Site pages
Current course
Participants
General
Module- 1 Engineering Properties of Biological Mat...
Module- 2 Physical Properties of Biomaterials
Module- 3 Engineering Properties
Module- 4 Rheological Properties of Biomaterials
Module- 5 Food Quality
Module- 6 Food Sampling
Module- 7 Sensory quality
Module 8. Quality Control and Management
Module 9. Food Laws
Module 10. Standards and regulations in food quali...
Lesson 32. Sanitation in food industry
Lesson 5. Basics of Thermal properties
Thermal properties are involved in almost every food processing operation. Knowledge of the thermal properties of foods is essential in the analysis and design of various food processes and food processing equipment involved in heat transport, with respect to heat transfer or energy use, such as in extrusion cooking, drying, sterilization, cooking etc. The most important thermal properties in food processing such as, specific heat capacity (cp), thermal conductivity (k), and thermal diffusivity of food materials depend mostly on the food’s composition, temperature and density. They have a significant effect on the rate of heat transfer into the particulates within the food product. When considering heat transfer during food processing, the thermal conductivity of the food plays an important role. The thermal conductivity (k) of food determines how fast heat can be evenly transferred to the entire food mass, which in turn affects the quality of the final product. Thermal conductivity depends strongly on moisture, temperature and structure of the material. In porous materials the void fraction and the pore structure and distribution affect thermal conductivity significantly. Generally, in multiphase systems (solids, water and air), the effect of geometric distribution of the phases is taken into account by using structural models.
|
5.1. Modes of heat transfer:
|
Fig. 5.1 Modes of heat transfer |
When heating and cooling of materials involves unsteady state or transient heat conduction, the material temperature changes with time and knowledge of the thermal diffusivity is required for predicting temperature in these processes.
The interface heat and mass transfer coefficients are important in the design of food processes and processing equipment, and in the control of food packaging and storage. Heat transfer coefficients are essential in thermal processing, in cooling or freezing and in storage operations. Mass transfer coefficients are important in drying and storage of foods, and in separation processes. One basic feature of both coefficients is that they are affected strongly by the characteristics of the processing equipment and the geometry of the system.
Conduction:- Heat transfer from one part of the homogenous opaque solid to another, under influence of the temperature gradient (transfer of heat by direct contact of particles of matter).
Heat is transfer by conduction when adjacent atoms brush against another or electron moves from one atom to another.
Steady state amount of heat in heat out = unsteady state.
Convection:- transfer of thermal energy by the movement of molecules from one part of the material to another.
Material convection
Forced convection
Radiation:- transfer of heat energy through empty space.
All object with a temperature above absolute zero radiate energy. No medium is necessary, for it is transferred through electromagnetic waves.
Related Physical Properties:-
Space, size, volume, unit surface area, roundness sphericity average projected area are need to be defined as quantified before heat transfer problems involving biological problems can be solved.
For example heat transfer through slab is different than that of sphere.
Specific heat:-
Heat required to raise the temperature by 1°C for unit mass of the body.
C =\[{q\over{m\Delta T}}\] q= heat, kcal
C = kcal/ kg°C m= mass, kg
\[\Delta\]T= temperature difference, °C
Thermal Conductivity:-
Thermal conductivity is defined as the amount of heat flows through unit thickness of material over an unit area per unit time for temperature difference.
Q=-k\[{{A\left({T1-T2}\right)}\over x}\times t\]
Ability to conduct heat: q= kA\[{{dt}\over{dx}}\]
Thermal conductivity is the quantity heat that passes through a body (plate) of particular area & thickness when it’s opposite faces difference in temperature.
q = kA\[{{dt}\over{dx}}\] t= Time, dT= temperature difference, dx= thickness
\[{{dQ}\over{d\theta}}\]= kA\[{{dt} \over{dx}}\] A= Surface area, k= thermal conductivity.
Heat transfer from higher to lower so (dT) accounts for the negativity (So final temperature will have lower value then the initial) to negate that (-) sign. The final equation for
‘k’=\[{{kcal-m}\over{m-hrk}}\] or
Thermal diffusivity:-
It is rate at which heat is diffused out of the materials. It is the ratio between thermal conductivity to volumetric heat capacity.
\[\alpha=\]m2/s=\[\alpha={k\over{PCp}}\]= PCp=Volumetric heat capacity
\[{{dt}\over{d\theta}}=\alpha\left({{{d2t}\over{dx2}}+{{d2t}\over{dy2}}+{{d2t}\over{dz2}}}\right)\]
Thermal Emissivity(E):-
It is a ratio of total Emissive power of a blackbody to the same temperature.
This thermal constant is associated with the rate of heat transfer by radiation from a hot body to a cold body.
Q = AFaFe\[\sigma\](T14-T24) E = 1 black body FaFe<1 real object
A= surface area
Fa = Angle factor, representing the angle that one body sees the other body.
Fe = Emissivity factor
\[\sigma\]= Stefan Boltzmann constant
T1 &T2 = Absolute temperature of body 1 and 2, respectively
While dealing with grey body Kirchhoff’s law of thermal radiation is applied emissivity equals to absorptivity (for an object in thermal equilibrium) so that the body does not absorb all incident light will also emit less radiation then ideal blackbody.
Stefan Boltzmann’s law:- The total energy radiated per unit surface area of a black body is unit time in proportional to the fourth power of the thermodynamic temperature.
\[\sigma\]=5.670410-8 W/ m2k-4
\[{P\over A}=\]\[\sigma\]T4 J/ m2s ____Stefan Boltzmann law
\[{P\over A}=E\[\sigma\]T4 Tc = Colder temperature
P = E\[\sigma\](T4 – Tc4) E= Emissivity
Thermal Effusively:- Square root of the product of the materials thermal conductivity & Volumetric heat capacity.
e = (KpCp)1/2 K= thermal conductivity
\[{{kal} \over {m2k\root {} \of {hr} }}\] \[\sqrt{{{kal}\over{S.m.c}}\times{{mg}\over{m^3}}\times{{kal}\over{kg^\circ C}}}\] p = density, Cp= rp heat
A material thermal effusivity is a measure of its ability to exchange thermal energy with its surrounding.
If two semi-infinite bodies initially at temperature T & T2 are brought in perfect thermal contact, the temperature of the contact surface Tm will be given by their relative thermal effusivity.
Tm= T1+(T2-T1)\[{{{\rm{e}}_{\rm{2}}}\over{\left({{\rm{e}}_{\rm{2}}+{\rm{e}}_{\rm{1}}}\right)}}\] (For semi infinite bodies in perfect thermal contact)
Diffusivity or diffusion constant is the proportionally constant between the molar flax due to molecular diffusion and the concentration gradient of the sphere.
Through Fick’s law (m2s-1)
Temperature dependence of the diffusion co-efficient
J =-D\[{{d\theta}\over{de}}\]\[\theta\]=Concentration gradient-D Ww= flow rate, A= surface area, y = Any position
D= diffusion Coefficient\[{{dc_W}\over{dy}}=\]change in cold water m2/s
Temperature Dependence
D=\[D_0e{{- E_a}\over{RT}}\] Arrhenius equation
Do = maximum diffusion coefficient at infinite temp.
Ea = activation energy
T = Absolute temp, K
R = universal gas constant
Thermal diffusivity is determines the speed of heat of the 3- dimensional propagation or diffusion through the material.
Mass transfer coefficient:-
It is the ratio of mass there of vapour (ww/A) at any point between the wet and dry surfaces to the difference in concentration of two boundary layers(lw0-lwc) between which mass is being transferred.
Kc=mass transfer coefficient,
Kc=\[{{n_A }\over{A\Delta c_A^{}}}\] \[\Delta c_A^{}\]=Conc. difference driving force, myl /m3
A=effective man transfer area, m2, nA=man transfer rate(mol / s)
Kc=
This is an analogy with heat transfer coefficient
Coefficient of Thermal expansion:- The change in size with temperature / C0
\[\alpha _V=\left({{1\over V}}\right)\left({{{dV}\over{dT}}}\right)\]V=Volumeofmaterial,\[{{dV}\over{dT}}\]=Rate of volume with temp.
\[{{\Delta T}\over V}=\alpha _VdT\]\[\alpha _V\]=Volumetric thermal expansion
Linear thermal expansion
\[\alpha _L={1\over L}{{dL}\over{dT}}\]
\[{{\Delta L}\over L}=\alpha _L LT\]
\[\alpha _V=3\alpha _L\]
\[\left({V+\Delta V}\right) =\left({L+\Delta L}\right)^3=L^3+3L^2\Delta L+3L\Delta L^2+\Delta L^2\L^3+3L^2\Delta\]
=\[V+3V{{dL}\over L}L^3+L^3\alpha _V\Delta T\]
=\[L^3+3L^3\alpha _L\Delta T+3L^3\alpha _L ^2\Delta T^2+L^3\alpha _L ^3\L^3 3L^3\alpha _L \Delta T\]
Since\[\Delta T\]is small the higher terms can be neglected.
\[\alpha _A={1\over A}{{dA}\over{dT}}\]
\[{{\Delta A}\over A}=\alpha _A \Delta T\]5
Table 5.1 dimensionless number used for calculation of thermal properties
|
Name |
Symbol |
Group of Physical properties |
|
|
Biot member |
Bi |
|
H=film heat coefficient or Convective heat |
|
Fourier number |
F0 |
|
Transfer coefficient |
|
Levies number |
Le() |
|
Lc= characteristic length |
|
Nusselt Number |
Nu |
|
K=Thermal conductivity of the body |
|
Prandlt number |
Pr |
|
Α=Thermal Diffusivity |
|
Regnolds number |
Rc |
|
=length though which conduction occurs |
|
Schmidt number |
Sc |
|
D= Man diffusivity |
|
Stanton number |
St |
|
|
Sc=\[{V\over D} = {\mu\over{PD}}\]
V= kinematic viscosity
\[\eta\left(\mu\right) = dynamicvis\cos ity\]
P= density
\[\Pr = {v \over \alpha } = {{\mu C_p }\over k}\]
Cp= specific heat
v= velocity
Nu=\[{{hL}\over{ks}}\]
St=\[{h\over{c_p pv}}\] h= convective heat transfer coefficient, P= density of fluid
St=\[{{Nu}\over{R_e .P_r }}\] Cp= sp heat, v= velocity of the fluid
Specific Heat calculation
Cavg= Cps= Sp. Heat of solid not Fat, Cpw= Sp. Heat of water
Cpw = 4186.8M, Cps=837.36
Cavg= 4186.8M+(1-M)837.36
Cavg=3349M+837.36 J /(ag.k)………….(1)
Cpf = Sp heat of fat = 1676.72
Cavg = 1674.72F + 7.36SNF + 4186.8M (J/ng0)………(2)
Energy Balances
Cp(above freezing point)=\[{{4.19M} \over {110}} + {{0.84(100 - M)} \over {110}}..........kJ/kg^o C\]
Cp(above freezing point)=\[{{2.1M} \over {110}} + {{0.84(100 - M)} \over {110}}\]
=\[335 \times {m \over {100}}\],
K =\[{{0.55M} \over {100}} + {{0.26(100 - M)} \over {100}}\]
Ku/Ou= \[{{2.4M} \over {100}} + {{0.26()100 - M} \over {100}}\]
The thermal properties of food materials depend on the composition of the food. Since most foods are composite of many macro and micromolecules, the thermal properties vary with the composition type. So the properties of food would vary than that of the individual pure molecules. In this context boiling point rise and freezing point depression of the foods are discussed in the following section.
Boiling point Rise:- In liquid food boiling point rise refers to water evaporation in which water change from the liquid phase to steam or vapour phase, and water vapour pressure equals to the external pre liquid foods contain high molecules weight solids that cause the boiling point be evaluated above that of pane water. The boiling point rise (\[\Delta T_r\]), is known as the increase in the boiling point over that of pure water in a given liquid food. As the vapour pressure of most aqueous solution is lower than that of water at the same temperature, the boiling temp(point) of the solution in higher than that of pure water.
Freezing point Depression: During freezing water in the food changes to ice which heat in removed by a refrigeration system. During heat removal, the unfrozen water will still contain dissolved solids. The presence of dissolved solids will depress the initial freezing point to certain amount \[\Delta Tf\] below the expected solidification temperature of pure water. Freezing point depression is defined as the temperature reduction \[\Delta Tf\].
Both the boiling point rise and freezing point depression of a food are related to its solution concentration.
Eutectic point:- Temperature where there no further concentration due to freezing , thus the solution frees. Temperature at which a crystal it indecision solute exists in equilibrium with unfrozen liquid rice. Crystallization occurs below eutectic point. The solution becomes super saturated.
A eutectic or eutectic mixture is a mixture of two or more phases at a composition that has lowest melting points & where the phases simultaneously crystallize from molten solution at this temperature. The proper ratios of phases to obtain a eutectic identified by the eutectic point on a phase diagram.
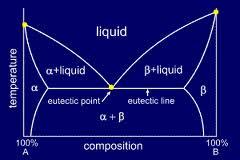
Fig. Eutectic point in a phase diagram
Frozen foods being thawed for are subjected to quality loss, if thawing is slow. Most concentrated solution that are last to freeze and slow to thaw, since they are eutectic mixture. The eutectic mixture is a solution of such composition that it frozen thaws as separation of pure ice. The frozen eutectic has a constant properties of ice crystal intermingled with solution crystal. The temperature at which a eutectic mixture is formed is called eutectic point or eutectic temperature. Freeze drying should be concerned out at this point. Maximum crystals formations is not possible until this temperature reached
Table 5.2 the eutectic point of different commodities
|
commodity |
Eutectic Temperature |
|
Ice cream |
55°C |
|
Meat |
50-60°C |
|
Bread |
70°C |
Application :
Freeze drying:- If eutectic point is not reached quickly the food have pure texture.
Thermal imaging: Infrared imaging for food quality determination
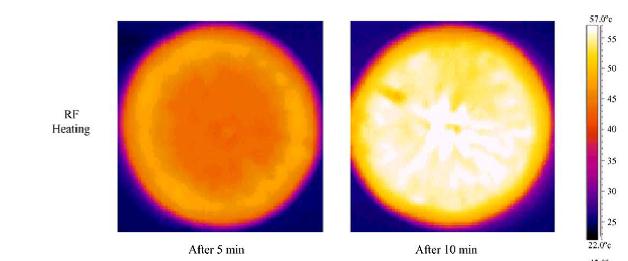
Fig. Thermal imaging of oranges when heated with radio frequency heating system (source: Birla et al., 2004)
References:
-
Fundamentals of food process engineering 3rd equation edited by Romeo T. Toledo, Springer.
-
S.L. Birla, S. Wang, J. Tang, G. Hallman. Improving heating uniformity of fresh fruit in radio frequency treatments for pest control. Postharvest Biology and Technology 33 (2004) 205–217