Site pages
Current course
Participants
General
Module 1. Moisture content and its determination.
Module 2. EMC
Module 3. Drying Theory and Mechanism of drying
Module 4. Air pressure within the grain bed, Shred...
Module 6. Study of different types of dryers- perf...
Module 5. Different methods of drying including pu...
Module 7. Study of drying and dehydration of agric...
Module 8. Types and causes of spoilage in storage.
Module 9. Storage of perishable products, function...
Module 10. Calculation of refrigeration load.
Lesson 3. Equilibrium Moisture Content (Emc) And Its Importance
3.1 Equilibrium Moisture Content (EMC)
Every food exerts a characteristic vapor pressure at a certain temperature and moisture content. All porous food materials, when in contact with moist air, adsorb or desorb water molecules to attain equilibrium moisture content. This equilibrium moisture content depends very strongly on the partial pressure of the water vapor in the surrounding air and rather weakly on the air temperature that are commonly experienced in drying and storage of foods. If the moisture content of the food material does not vary with time for a given combination of water vapor pressure and air temperature, it is then said that it has reached the equilibrium moisture content (EMC) of the material at that water vapor pressure and temperature. When left undisturbed, the natural end of any adsorption or desorption process is the attainment of EMC. This, though a natural process, is often very slow. Food materials may take several days or weeks to reach equilibrium.
When the water vapor pressure of the air approaches the saturation water vapor pressure at the temperature of the air, the EMC of food materials rapidly increases. At these stages, the process undergone by the food material is not only adsorption. Water vapor begins to condense within the pore structures of the building materials. Theoretically, if the food material is in contact with air that is 100 % saturated for a very long period, all pores of the material should be filled with the condensed moisture. The EMC that corresponds to that hypothetical state is called the saturation moisture content of the material. But in practice the rate of this process becomes infinitesimally small at an EMC that is known as the capillary saturation moisture content and is often substantially less than the saturation moisture content referred to above.
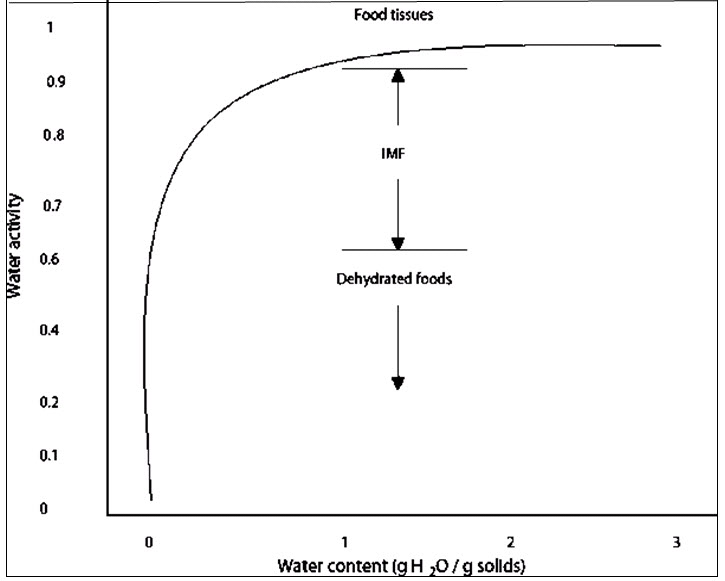
Fig 1. Relationship between equilibrium moisture content and water activity
Drying of grain involves exposing grain to ambient air with low relative humidity or to heated air. This will evaporate the moisture from the grain and then the drying air will remove the moisture from the grain bulk. Since drying practices can have a big impact on grain quality or seed quality, it is important to understand some fundamentals of grain drying.
3.2 Importance of Equilibrium Moisture Content
The concept of equilibrium moisture content (EMC) is important in the study of drying and storage of fruits, vegetables and grains. The EMC helps to decide the stability of food at particular moisture content in the given environment. The EMC values of several grains and foods have been determined by several research workers and reported in literature. If exposed to air, high moisture foods, loose moisture whereas low moisture foods gain moisture in humid air. EMC determines the minimum moisture content to which food can be dried under a given set of conditions. Also, it determines the maximum amount of moisture the dehydrated food can absorb during storage.
The EMC can be elaborated by an example given by Hall (1980). If wheat and oats having 16 % moisture content are kept in the environment of 86°C and 75% relative humidity, wheat will absorb moisture and oat will lose. This is because of the different vapor pressures of the moisture in wheat and oat. At above air temperature and humidity the vapor pressure of wheat is 0.444 psi and oat is 0.477 psi where as vapor pressure of moisture in air at 86°C and 75% relative humidity is 0.461 psi. Therefore, wheat will gain moisture from air as vapor pressure of air is more than wheat. At the same time oat will lose moisture as its vapor pressure is higher than air. There is always tendency of movement of moisture from higher vapor pressure to lower vapor pressure. The Figure 2 shows the movement of moisture from and to food.
Figure 2. Movement of moisture from and to food.
When the vapor pressure of the water held by food particles becomes equal to the water vapor pressure of the surrounding air the movement of moisture stops and comes to equilibrium. At this moment the food product remains at the same moisture content in the same air temperature and humidity. This moisture is called as equilibrium moisture content. The relative humidity of air surrounding the food particles at the same conditions is called as equilibrium relative humidity (ERH) which is also known as water activity (aw).
3.3 Factors affecting the EMC
The EMC is dependent mainly on:
1. Air temperature
2. Relative humidity.
If a food sample of same initial moisture content and vapor pressure is exposed to air having different vapor pressures, it will come to equilibrium at different moisture content values (EMCs). Therefore, it can be understood that EMC is dependent on the air vapor pressure which in turn depends on the temperature and relative humidity of air. Figure 3 shows the graphical representation of change of EMC with different vapor pressures (V1 to V4). For example a shelled corn at air having 70% relative humidity and 40 °F, EMC is 15.7% whereas it is 10.3% at 140°F. A change in the chemical composition can affect the EMC of food products. Foods with high oil content adsorb less moisture from the surrounding air. Also, previous moisture adoption desorption history of food affects EMC values. Plotting different EMC values with relative humidity at constant temperature results in sigmoid type (S-shaped) curve.
Figure 3. Graphical representation of change of EMC with different vapor pressures (V1 to V4)