Site pages
Current course
Participants
General
MODULE 1. Magnetism
MODULE 2. Particle Physics
MODULE 3. Modern Physics
MODULE 4. Semicoductor Physics
MODULE 5. Superconductivty
MODULE 6. Optics
5 April - 11 April
12 April - 18 April
19 April - 25 April
26 April - 2 May
LESSON 25. Basic concept of LASER
LASER
No other scientific discovery of the 20th century has been demonstrated with so many exciting applications as laser acronym for
Light Amplification by Stimulated Emission of Radiation.
The basic concepts of laser were first given by an American scientist- Charles Hard Townes and Soviet scientists- Alexander Mikhailovich Prokhorov and Nikolai Gennediyevich Basov
-
Laser is a powerful source of light having extraordinary properties which are not found in the normal light sources like tungsten lamps, mercury lamps, etc. The unique property of laser is that its light waves travel very long distances with every little divergence.
-
In case of a conventional source of light, the light is emitted in a jumble of separate waves that cancel each other at random
Fig.16(1) and hence can travel very short distances only.
- On the other hand, if a small stones are thrown into a pool one by one at the same place and also at constant intervals of time, the waves thus generated strengthen each other and travel long distances. In this case, the waves are said to travel coherently. In laser, the light waves are exactly in step with each other and thus have a fixed phase relationship
It is this coherency that makes all the difference to make the laser light so narrow, so powerful and so easy to focus on a given object. The light with such qualities is not found in nature.
Basic Concept of the LASER
Absorption
- Let the atom is in the lower energy state (E1).
- Now, a photon of energy (E1 - E2) is incident on the atom at lower energy state.
-
Photon gives its energy to the atom and disappeared.
-
Then we can say that atom absorbed the incident photon.
-
Due to sufficient absorption of energy by the atom, the atom jumps to the exited state (E2) from lower energy state (E1).
-
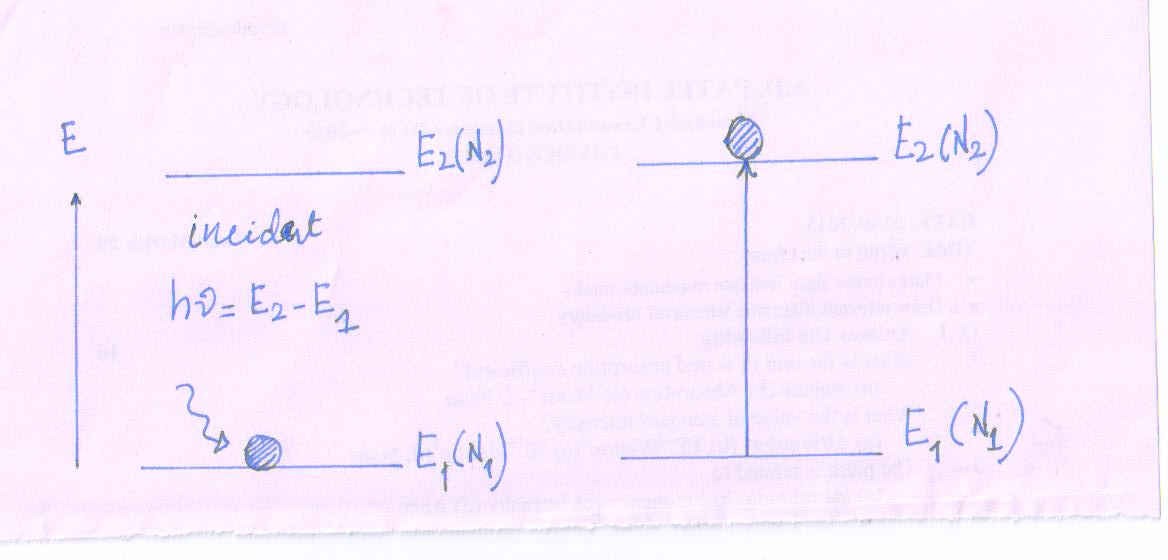
Fig16(3)
This is called absorption process and corresponding transition is called Absorption Transition.
Due to sufficient absorption of energy by each absorption transition at event an atom is excited and one photon is subtracted from the incident beam of light (bunch of incident photon)
So we can expressed the equation for absorption process
A + hν = A*
Where, hν - incident photon energy
A - An atom in lower energy state (E1)
A* - An atom in excited state (E2)
Rate of absorption transition (R12) occur is proportional to the incident photon density ρ(ν) and number of atom are in lower energy state (E1) at Δt
R12 \[ \propto\] ρ(ν)
R12 \[ \propto\] N1
Hence,
R12 = B12N1 ρ(ν) Δt…..(1)
R12 - Rate of absorption transition
N1 - Number of atoms in lower energy state.
ρ(ν) - Incident photon density.
B12 - Constant of proportionality; known as Einstein coefficient for induced absorption
Δt - Time duration for absorption (excitation) process.
Here, N1 is the population (more numbers of atoms) of atom at E1, when more number of atoms are at lower energy state(E1), then more atoms may jump into excited state (E2). Further, when more photons are incident on the system, then more atoms may get excited to the higher energy level.
Normally, number of atoms in higher energy state (N2) can’t be greater than number of atoms in lower energy state (N1). But, induce absorption involves the excitation of atom to the definite higher energy level (hν = ΔE). As a result,N1 decreases and N2 increases.
Spontaneous Emission
-
After the completion of the absorption process (transition), the atom in the excited state (E2) can’t stay in the excited state for a relative long time.
-
The time of about 10-8 sec, the atom returns to the lower energy state (E1) by releasing the photon of energy which was gained during absorption process. i.e hν = E2 - E1
-
The emission of photon occurs on itself and without any external energy (force) to the excited atom. Hence, emission of photon by an atom without any external energy is called spontaneous emission.
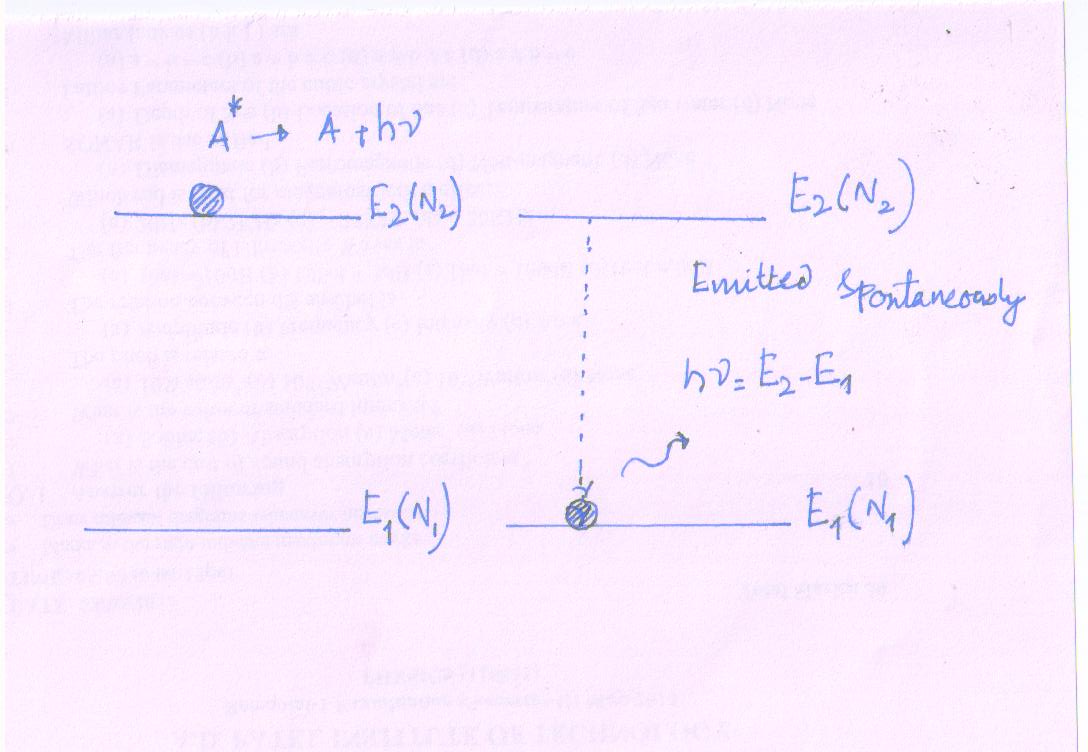 Fig16(4)
Fig16(4)
Expression for spontaneous process as
A* → A + hν
Due to absence of external energy; the rate of spontaneous (R21) occurs depends upon the properties of energy states E2 and E1 only.
It is independent of photon density.
R21 \[ \propto\] N2
R21 = A21 N2 Δt ……….. (2)
N2- Number of atoms in excited state (E2)
A21- Constant of probability; Einstein coefficient for spontaneous emission.
Δt - Time duration for spontaneous transition.
It is a process of spontaneous emission that dominates (have a very strong influence over) in conventional light source.
Stimulated Emission
-
Einstein reports to establish of equilibrium between matter and radiation required a new radiation process.
-
It is called stimulated radiation process.
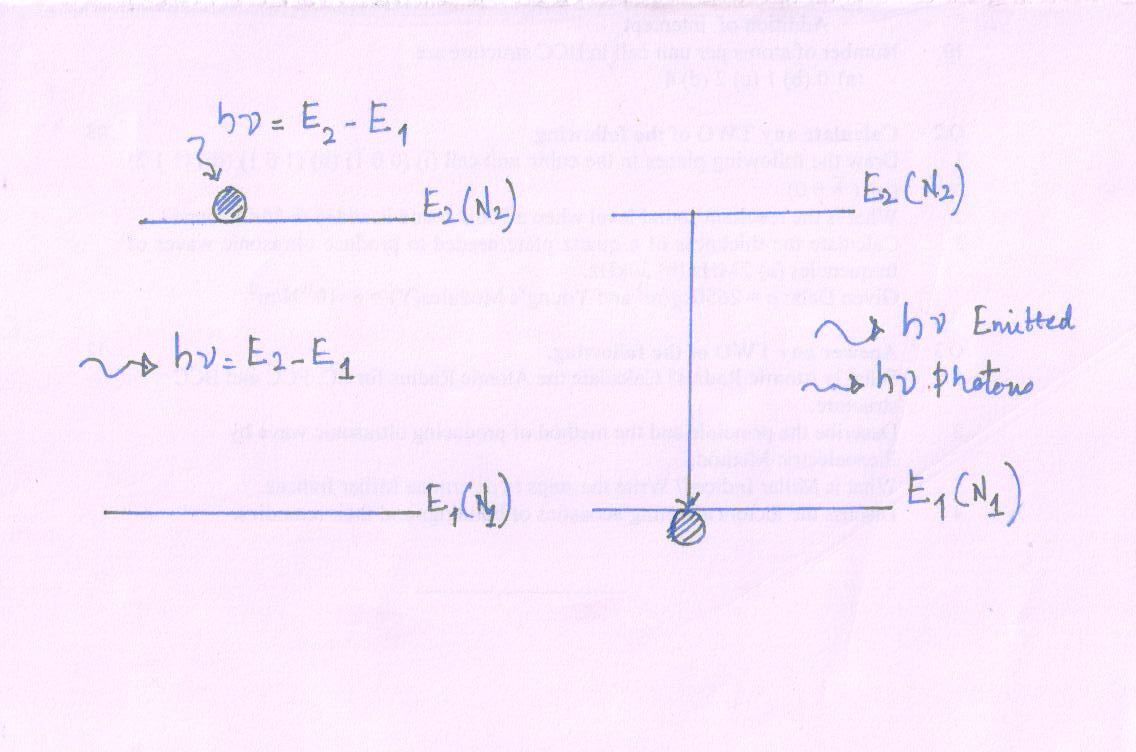
Fig16(5)
- It requires external radiation.
Let an atom in the excited state interact with a photon (external radiation) with energy hν = E2 - E1, an external photon induces (bring out) the excited atom to make a downward transition. But such downward transition well before the atom can make a spontaneous transition.
The atom emits the excess energy in the form of a photon; hν = E2 - E1 as it drops to lower energy state. Phenomenon of force photon emission by an external atom due to the role of external agency is called stimulated emission. The stimulated emission process may be expressed
A* + hν (external) → A + 2hν
Rate of stimulated transition is proportional to external incident photon density ρ(ν) and the number of atom at energy level E2 at Δt
R21 \[ \propto\] ρ(ν) N2 Δt
R21 = B21 ρ(ν) N2 Δt …….. (3)
B21 - Constant of probability; known as Einstein coefficient for stimulated emission.