Site pages
Current course
Participants
General
Module 1. Micro-irrigation
Module 2. Drip Irrigation System Design and Instal...
Module 3. Sprinkler Irrigation
Module 4. Fertigation System
Module 5. Quality Assurance & Economic Analysis
Module 6. Automation of Micro Irrigation System
Module 7. Greenhouse/Polyhouse Technology
12 April - 18 April
19 April - 25 April
26 April - 2 May
Lesson 18. Fertigation
18.1 Introduction
Fertigation is precise application of plant nutrients with irrigation system in the crop root zone according to the crop demand during crop growing season.
In fertigation, fertilizer application is made in a small and frequent dose that feed within scheduled irrigation interval matching the plant water use to avoid leaching. The right combination of water and nutrients is the key for high yield and quality of produce. Table 18.1 provides details of saving in the use of fertilizers and increase in yield.
Table: 18.1 Saving in fertilizer and increase in crop yield due to fertigation as compared to conventional method of fertilizer application
|
S. No. |
Crop |
Saving in fertilizer, % |
Increase in yield, % |
|
1 |
Okra |
40 |
18 |
|
2 |
Onion |
40 |
16 |
|
3 |
Broccoli |
40 |
10 |
|
4 |
Banana |
20 |
11 |
|
5 |
Castor |
60 |
32 |
|
6 |
Cotton |
30 |
20 |
|
7 |
Potato |
40 |
30 |
|
8 |
Tomato |
40 |
33 |
|
9 |
Sugarcane |
50 |
40 |
(Source: Anonymous, 2001)
18.2Need of Fertigation
It has been reported that Indian soils are deficient in nutrients. In order to achieve high crop production it is required to supplement nutrient by adding chemical fertilizers and nutrients. These fertilizers are expensive. Large quantities of fertilizers are imported to meet the growing demand. On the other hand country is facing low fertilizer utilization efficiency hence there is need for using fertigation system.
Fertigation has been seen as a tool that can help to accomplish the goals of agronomic and aesthetic lushness with relative ease. It enhances the efficiency of meeting the nutritional needs of plants. Fertigation allows the selection of nutrient inputs as needed or whatever fits the “prescription” to match stage of growth, time of year, as well as saving in operating money. It is a safer application method, as it eliminates the danger of affecting roots due to higher dose.
18.3Advantages and Limitations of Fertigation
Advantages
-
Synchronization with plant requirement: In drip fertigation, fertilizer application is synchronized with plant need which varies from plant to plant. In drip fertigation, the amount and form of nutrient supply is regulated as per the need of critical stages of plant growth.
-
Economics: Saving in amount of fertilizer, due to better fertilizer use efficiency and reduction in leaching. Reduction in labour and energy cost by uniform water and nutrient distribution.
-
Balanced nutrient availability: Optimization of nutrient balance in soils by supplying the nutrients directly to the effective root zones as per the requirement.
-
Higher yield: Greater yield and quality of produce is obtained.
-
Higher fertilizer use efficiency: Ensures a uniform flow of water and nutrients. Timely application of small but precise amounts of fertilizers directly at the plant roots zone, this improves fertilizer use efficiency and reduces nutrient leaching below the root zone. Improves availability of nutrients and their uptake by crop.
-
Safer application method, as it eliminates the danger affecting roots due to higher dose.
Limitations
-
High initial investment: The drip and fertigation equipment components are expensive.
-
Relatively higher maintenance cost: The maintenance cost of drip and fertigation system is higher.
-
Clogging of drip emitter: Good quality water is very essential. Due to precipitation of chemicals, clogging of drip emitters may cause a serious problem.
-
Availability of water soluble fertilizers& its compatibility: It needs water soluble fertilizers, the availability of these types of fertilizers is limited. Adjustment of fertilizers to suit the need is not easy.
-
Subsidy in drip system: Area under micro irrigation is increasing mainly because of subsidy in micro-irrigation, if subsidy is withdrawn, the area under micro-irrigation may also reduce. So same may be the fate of fertigation.
-
Overdosing: Due to fear of yield loss, because of relatively lower dose of fertilizers in fertigation, farmers have the tendency to add additional fertilizers and irrigation water by traditional methods too. This may result in crop loading (sugar cane) lower yield and lower profits.
18.4 Types of Fertilizers
A large range of fertilizers, both solid and liquid, are suitable for fertigation depending on the physicochemical properties of the fertilizer solution. For large scale field operations, solid fertilizer sources are typically a less expensive alternative to the commonly used liquid formulations. The solubility of these fertilizers does vary greatly.
The main fertilizers/chemicals used for fertigation are:
Nitrogen: Nitrogen is usually applied through the system as anhydrous ammonia, aqua ammonia, ammonium phosphate, urea, ammonium nitrate, calcium nitrate or several other mixtures. Careful consideration must be made for the pH in irrigation water since some nitrogen sources, particularly aqua ammonia and anhydrous ammonia will increase pH. The increased pH can result in precipitation of insoluble calcium and magnesium carbonates that can clog the drip system. Urea and urea-ammonium nitrate mixture are highly soluble and usually do not cause large pH shifts.
Phosphorus: Phosphoric acid is soluble and with low pH water has no clogging problems. Sulfuric acid injection together with phosphoric acid may be sufficient to prevent precipitation of calcium and magnesium especially as the phosphoric acid boundary passes. Inorganic phosphate, orthophosphate and glycerophosphate have also been used to supply phosphorus.
Potassium: Potassium can be applied as potassium chloride and potassium nitrate. These potassium sources are soluble and have few precipitation problems. The Potassic fertilizers are water soluble and quick acting such as potassium chloride or muriate of potash, potassium sulphate, potassium magnesium sulphate, also known as Sulphate of potash magnesia.
The K ions are absorbed in the soil and thus remain available, and largely protected against leaching. However, split application is advisable where higher leaching losses may be expected. Some immobilization into clay lattice layers reduces availability but strong fixation into completely unavailable forms is limited to a few special soil types.
All types of fully-water soluble granular and liquid fertilizers are suitable for fertigation. However, for higher yield and quality, chloride-free fertilizers such as Multi-K (potassium nitrate), Mono ammonium Phosphate and Mono Potassium Phosphate are preferred. Soluble dry fertilizers containing N, P and K in different combinations are also available in the market.
Micronutrients:Manganese, zinc,iron, copper, etc., may be applied as soluble salts through the irrigation system. These should be injected separately and apart from other fertilizers and chemicals to avoid chemical interaction and precipitation. Iron, copper, zinc and manganese may react with salt in irrigation water and result in precipitation. However, the more soluble chelated forms such as iron or zinc EDTA (ethyl-enediaminetetraacetatedidhydrate) usually cause little clogging problem.
18.5 Fertilizers solubility and their compatibility
Solubility of Fertilizers
The quantity of fertilizer that can be dissolved in unit quantity of water is called the solubility. Normally nitrogen and potassic fertilizers do not have solubility problem.However, phosphatic fertilizers such as DAP & SSP do not readily dissolve in water. The solubility is greatly affected by the temperature variations. The solubility decreases with decrease in temperature. Table 18.2 provides the solubility limit (g/l) of nitrogenous, potassic and phosphatic and micro nutrient fertilizers.
Table: 18.2a Solubility of Nitrogenous Fertilizers
|
Types of Fertilizer |
Nitrogen content(%) |
Solubility(g/L) |
|
Ammonium Sulphate |
21 |
750 |
|
Urea |
46 |
1100 |
|
Ammonium Nitrate |
34 |
1920 |
|
Calcium Nitrate |
15.5 |
1290 |
Table: 18.2b Characteristic of Nitrogenous Fertilizers Suitable for Fertigation
|
Fertilizers |
Grade |
Formula |
pH(1g/L at 200C) |
|
Urea |
46-0-0 |
CO(NH2)2 |
5.8 |
|
Potassium Nitrate |
13-0-46 |
KNO3 |
7.0 |
|
Only Fertigation grade |
|||
|
Ammonium Sulphate |
21-0-0 |
(NH4)2SO4 |
5.5 |
|
Urea Ammonium nitrate |
32-0-0 |
CO(NH2)2.NH4NO3 |
|
|
Ammonium Nitrate |
34-0-0 |
NH4NO3 |
5.7 |
|
Mono Ammonium Phosphate |
12-61-0 |
NH4H2PO4 |
4.9 |
|
Calcium Nitrate |
15-0-0 |
Ca(NO3)2 |
5.8 |
|
Magnesium nitrate |
11-0-0 |
Mg(NO3)2 |
5.4 |
Table: 18.2c Solubility of PotassicFertilizers
|
Fertilizer |
K content (%) |
Solubility (g/L) |
|
Potassium Sulphate |
50 |
110 |
|
Potassium Chloride |
60 |
340 |
|
Potassium Nitrate |
44 |
133 |
Table: 18.2d Characteristic of Potassic Fertilizers Suitable for Fertigation
|
Fertilizers |
Grade |
Formula |
pH(g/L at 200C) |
Other Nutrients |
|
Potassium Chloride@ |
0-0-60 |
KCl |
7.0 |
46% Cl |
|
Potassium Nitrate |
13-0-46 |
KNO3 |
7.0 |
13% N |
|
Potassium Sulphate# |
0-0-50 |
K2SO4 |
3.7 |
18% S |
|
Potassium Thiosulphate* |
0-0-25 |
K2S2O3 |
- |
17% S |
|
Monopotassium Phosphate |
0-52-34 |
KH2PO4 |
5.5 |
52% P2O5 |
@ only white, # only Fertigation grade, * liquid.
Phosphatic Fertilizers:
P + Ca = Calcium phosphate (insoluble).
P +Ammonium + Magnesium = Magnesium ammonium phosphate (insoluble).
P + Iron = Iron phosphate (insoluble).
Even good P sources like poly phosphates get precipitated if Ca + Mg in water is> 50ppm and Bicarbonate > 150ppm. If Bicarbonate content is < 100ppm, the Ca + Mg content can go upto 75ppm.
Table: 18.2e Characteristics of Phosphorus Fertilizers suitable for fertigation
|
Fertilizers |
Grade |
Formula |
pH (1g/ L at 200C) |
|
Phosphoric acid |
0-52-0 |
H3PO4 |
2.6 |
|
Mono- potassium Phosphate |
0-52-34 |
KH2PO4 |
5.5 |
|
Mono ammonium phosphate |
12-61-0 |
NH4H2PO4 |
4.9 |
Table: 18.2f Solubility of Micro-nutrient Fertilizers
|
Fertilizer |
Content (%) |
Solubility (g/L) |
|
Solubor |
20B |
220 |
|
Copper Sulphate |
25 Cu |
320 |
|
Iron Sulphate |
20 Fe |
160 |
|
Magnesium Sulphate |
10 |
710 |
|
Ammonium Molybdate |
54 |
430 |
|
Zinc Sulphate |
36 |
965 |
|
Manganese Sulphate |
27 |
1050 |
(Source: http://www.ncpahindia.com/articles/article17.pdf; Oct 09, 2012)
Compatibility
Mixing the solutions of two or more than two water soluble fertilizers can sometimes result in the formation of a precipitate. Their solutions should be prepared in two separate tanks. Table 18.3 gives compatibility chart of different water soluble fertilizers.
Interaction between fertilizers nutrients-compatibility
When preparing fertilizer solution, the solubility product of the different materials must be taken into consideration. The solubility of the mixture fertilizer, getr required due to formation of precipitates:
a) Calcium nitrate with any sulphates= formation of CaSO4 precipitate (gypsum)
Ca(NO3)2 + (NH4)2SO4 ◊ CaSO4↓ + ………….
b) Calcium nitrate with any phosphates = formation of Ca phosphate precipitate
Ca(NO3)2 + NH4PO4 ◊ CaHPO4 ↓ +…………
c) Magnesium with mono-ammonium phosphate = formation of magnesium phosphate precipitate
Mg(NO3)2 + NH4H2PO4 ◊ MgHPO4 ↓ + ………...
d) Ammonium Sulphate with KCl or KNO3 : formation of K2SO4 precipitate
SO4(NH4)2 + KCl or KNO3 ◊ K2SO4 ↓ + ………..
e) Phosphorus with iron = formation of iron phosphates precipitate.
Table: 18.3 Compatibility chart for different water soluble fertilizers
|
Fertilizers |
Urea |
Ammonium Nitrate |
Ammonium Sulphate |
Calcium Nitrate |
Mono Ammonium Phosphate |
Mono Potassium Phosphate |
Potassium Nitrate |
|
Urea |
|
C |
C |
C |
C |
C |
C |
|
Ammonium Nitrate |
C |
|
C |
C |
C |
C |
C |
|
Ammonium Sulphate |
C |
C |
|
LC |
C |
C |
LC |
|
Calcium Nitrate |
C |
C |
LC |
|
NC |
NC |
C |
|
Mono Ammonium Phosphate |
C |
C |
C |
NC |
|
C |
C |
|
Mono Potassium Phosphate |
C |
C |
C |
NC |
C |
|
C |
|
Potassium Nitrate |
C |
C |
LC |
C |
C |
C |
|
C=COMPATIBLE, NC=NOT COMPATIBLE, LC=LIMITED COMPATIBLE.
Table: 18.3b Fertilizer evaluation for suitability to fertigation
|
Property |
NH4NO3 |
(NH4)2SO4 |
K2SO4 |
KCl |
KNO3 |
H3PO4 |
MAP |
|
Solubility |
High |
Medium |
Low |
Medium |
Medium |
High |
Medium |
|
Precipitation |
Low |
High |
High |
Low |
Low |
Low |
High |
|
Compatibility |
Good |
Poor |
Poor |
Medium |
Medium |
Medium |
Good |
|
Corrosion |
Medium |
Poor |
Poor |
Poor |
Good |
Poor |
Medium |
(Source: http://www.ncpahindia.com/articles/article17.pdf)
18.6 Safety precautions and back flow prevention
Fertigation safety devices
Backflow can occur in a system due to cross connection between a water source and an irrigation system. For example, water may be turned off, but the chemical injection unit may continue to work, which contaminate the water source. To protect groundwater and drinking water supplies from chemical contamination, backflow – whether from backsiphonage or backpressure – must be prevented. The main chemigation safety devices which are used to prevent backflow are shown in the Fig 18.1.
Backsiphonage is the reversal of normal system flow, caused by negative pressure (vacuum or partial vacuum) in the supplying pipe. Backsiphonage occurs due to low pressure in the water source. For example, the mainline source pipe may break at a spot lower than the irrigation system or pressure may be reduced drastically because a supply pump fails. Such situations can be avoided by installing check valves, vacuum relief valves or vacuum breaker valves.
Backpressure is the reversal of normal system flow due to downstream pressure increasing above supply pressure. Backpressure may occur if a system operates at higher pressures than its water supply, perhaps due to use of booster pumps or interconnection of a water source to other water systems. Such situations can be avoided by installing double check valves or special valves that combine check valves with reduced pressure zones commonly known as reduced pressure principle backflow prevention valves (Encisoand Porter, 2005)
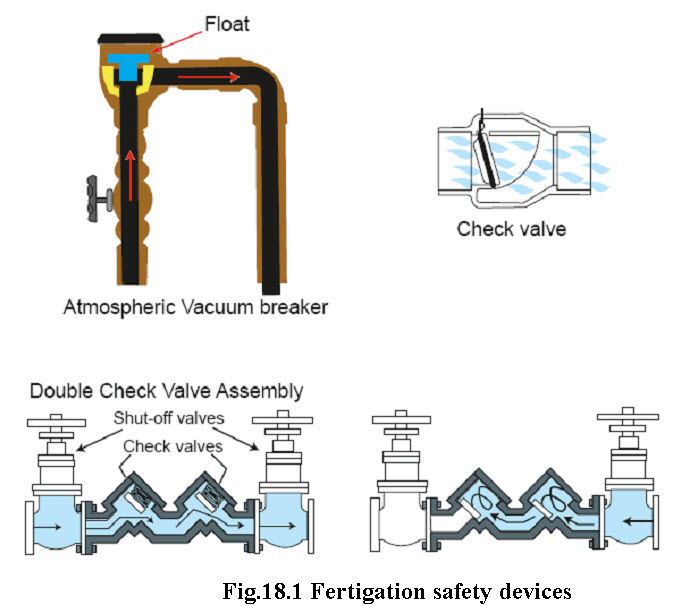
(Source: Encisoand Porter, 2005)
The following safety precautions should be observed for successful fertigation system.
Operating pressure variations should be minimum in order to achieve uniform mixing of nutrients and irrigation water in the drip irrigation system.
Compatibility of two fertilizers should be checked to avoid precipitation that may cause clogging of drip emitters and filter system.
To eliminate entry of solid particles or undissolved fertilizers a small screen/ strainers should be placed at the end of suction line.
Adjustment of the water pH should be made as per the pH of fertilizer solution.
Pesticides and chlorine should not be injected in combination with fertilizer solution.
To remove undissolved fertilizer particles or precipitates, the fertilizer injection point must be at the upstream end of the filter system so that the adequate filtration can be made.
Irrigation and fertilizer injection pumps should be compatible to prevent entry of fertilizer in the irrigation line. The controlfor the motors of both the pumps should be electronically interlocked.
Check valves should be installed in the fertigation injection line to prevent the back flow of water from irrigation system into fertilizer supply tank.
Check and vacuum relief valves should be installed to prevent water or mixture of water and fertilizer from draining or siphoning back into water source.
References
Anonymous, (2001). National Committee on Plasticulture Application in Horticulture, Progress Report-2001, Department of Agriculture and Co-operation, Ministry of Agriculture, GOI, New Delhi, 98p.
Enciso, J. and Porter.D. (2005).Basics of Micro-irrigation.Texas Cooperative Extension.Weslaco and Lubbock, Texas.Publication Number B-6160.
Michael, A. M. (2010).Irrigation Theory and Practice, Second Edition, Vikas publishing House, Noida, U.P.
http://en.wikipedia.org/wiki/Fertigation, Oct 07, 2012.
http://www.ncpahindia.com/articles/article17.pdf, Oct 11, 2012.
Suggested Reading
James L. G. (1988). Principles of Farm Irrigation System Design, USA. pp. 297.