Site pages
Current course
Participants
General
Module 1. Introduction to by-products and waste ge...
Module 2. Waste management concepts
Module 3. Direct combustion of solid waste
Module 4. Thermo-chemical conversion of solid waste
Module 5. Bio-chemical conversion of solid waste
Module 6. Solid waste management
Module 7. Effluent treatment and disposal
Module 8. Presence of typical chemicals
19 April - 25 April
26 April - 2 May
Lesson 14.
Tertiary or advanced treatment pertains to the unit operations and chemical unit processes which are used to further remove BOD, nutrients, pathogens, parasites and sometimes toxic substances. These include both physical and chemical processes, like filteration using sand, coal or activated carbon filters or chemical oxidation processes which utilizes chlorination, H2O2 fentons reagent, ozone and permanganate.
The physical oxidation processes which include
-
UV oxidation
-
Sonication
-
Wet air oxidation
- Supercritical water oxidation (SCWO)
Filteration
Filteration is defined as the process of allowing waste water to pass through a thick layer of sand or other filtering media. Filters may be classified as slow sand filters and rapid sand filters depending upon the rate of filteration. The phenomena of filteration is quite complex, but generally four function are performed.
-
Mechanical straining: which removes large suspended matterwhich cannot pass through interstics between sand grains.
-
Sedimentation and adsorption: accounts for the removal of colloids, suspended and bacterial particles. The interstices between sand grains act as minute sedimentation basins.
-
Biological metabolism: it is the growth and life process of the living cells. The surface layer gets coated with a zoogleal film in which bacterial activities are highest.
-
Electrolyte action: a certain amount of dissolved and suspended matter in H2O is ionized which interacts with particles of sand which are also ionized. These neutralize each other and while doing so, change the chemical character of water.
The selected filter sand should be free from clay, loam, vegetable or organic matter. It should also be of uniform and of proper size. If the sand is too fine, it tends to quickly clog which causes a greater loss of head in the filter.
If the sand is too coarse, it will permit suspended solids and bacteria to pass through voids between the sand grains. Effective size of the sand is defined as the sieve size in mm which permits 10% of the sand by weight to pass or in other worlds, as the size of the grain that is larger than 10% by weight of all the particles comprising the sand.
Construction of slow sand filter
It consists of a water tank, 2.5-3.5m in depth, having a sand bed 0.6-0.9m thick, supported on a bed of gravel 0.3-0.45m thick laid in 5-6 layers, beneath with the under drainage system is laid over a concrete bed sloping towards a central longitudinal drain. The underground system consists of open-jointed drains of baked clay or concerete pipe in length of 30-40m which a maximum spacing of lateral of 2m.
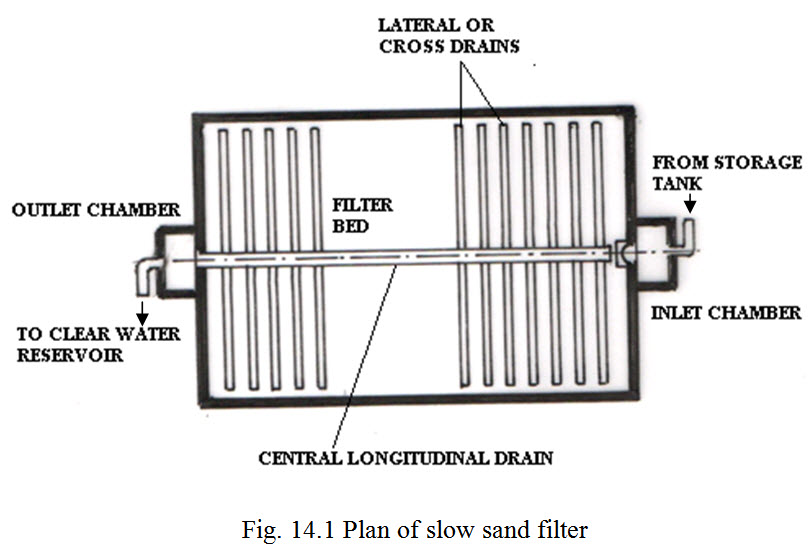
The raw mater is led gently on the filter bed, percolating downwards passes through the underdrains iinto an outlet chamber.
Construction of rapid sand filter (Gravity type)
It consist of an oper water-tight tank 3-3.5m deep of masonry or concrete with a concrete floor having coarse sand 0.6-0.75m thick, laid on the top with a layer of grated gravel 0.6-0.75m thick, laid on the top with a layer of grated gravel 0.45m thick supporting below. The gravel is underlain by an underdrainge system consisting of cast iron, central longitudinal conduct or manifold with strainers mounted on top and pipes of smaller diameter called lateral branching off at right angle to the manifold. The laterals are fixed 15-30cm centres and carry perforations on sides and bottom. About 1.75-2.5m of water depth may be allowed on the filter bed.
Comparative characteristics of slow sand filter and Rapid Sand filters
|
Characteristic |
Slow Sand Filter |
Rapid Sand Filter
|
|
Area |
Require more area |
Require less area |
|
Size of sand or filtering media |
Sand grains are finer. Effective size lies between 0.3-0.35mm Uniformly coefficient is 2. |
Sand grains slightly coarser. Effective size lies between 0.45-0.70mm Uniformly coefficient is 1.70 maximum. |
|
Rate of filteration |
Low 100 to 200 litres/m2/hr. |
High 2000 to 6000 litres/m2/hr. |
|
Loss of head |
It is very small. Only 0.6-0.9m |
It is more -2.5 to 3m. |
|
Supervision |
Simple to operate. Does not require Skilled operators |
Requires skilled operators. |
|
Coagulation |
Does not require premliminary coagulant mixing. |
Requires the addition of a coagulant in order to get satisfactory filteration. |
|
Method of cleaning |
Washing is done by scrapping a thin layer of sand from the surface. |
Washing is done by reversing the flow of water, taking the help of compressed air and a high pressure of wash water. Washing is completed in a few minutes, say 15-30 minutes. |
|
Period of cleaning |
Once every 2-3 months |
24-48 hr |
|
Amount of wash water |
0.2-0.6% of filtered water. |
2-4% of filtered water. |
|
Efficiency |
Remove bacteria completely because the rate of filteration is slow |
Remove bacteria only partially. It is more efficient in the removal of colour. because the rate of filteration is slow |
|
Operating Cost |
Operating cost per thousand litres of water treated is less. |
It is more |
|
Maintenance cost |
Small |
More because of maintenance of pumps for pumping up wash-water and a compressor unit for obtaining compressed air. |
Drawbacks of rapid sand fillers
Due to filetration on graded sand the majority of impurities are removed and stored in the limited space available in top sand layers. As the filters gets stratified after back washing it leads to surface clogging with quicker build up of head loss at higher velocity of filteration. Therefore the rapid sand filter has to be operated at somewhat lower filteration rates with shorter filter runs. The fine to coarse gradation of filter medium often results in poor filterate quality due to non-removal of finer-floc particles which may lodge in the region below the top sand layers.
To offset these drawbacks, indepth filteration is done where impurities laden water first passed through a medium having maximum pore space for arresting impurities. After this, the resulting water travels deeper into filter beds come in contact with the layers having smaller pore size. This enables the smaller sized particles to be detained and finally removed along with the finer floc particles.
This leads to higher quality filterates and greater utilization of the lower filter bed layers and consequently increased filter runs. This concept of indepth filteration uses the following filters:
1. Dual media filters
A dual media filter consists of a fine sand layer topped with a bed of anthraute coal each generally 30cm deep. The coarser an thracite top layer has intustices about 20% larger than the sand media. These openings are capable of adsorbing and trapping particles so that the flocs carried once in clarifier does not accumulate on the filter surface and sand bed forematurily. Because of the different specific gravity f the two materials (Coal 1.4 and sand 2.65), the coal of proper size in relation to the sand remains on top of sand during back washing.they have high rate of filteration of about 12000-15000 litres/m2/h with the produce of high quality water. However, due to low total surface area of media particles they have a low resistance to turbidity break-through with changing flow rates.
2. Mixed media filter
A mixed media filter is a three layer filter of three properly graded materials: Coal (sp. gr.1.4), sand (sp.gr.2.65) and garnet (sp.gr.4.2) in the proporation of 60, 30 and 10% resp. the particle sizes range from 1.0mm to 0.12mm from top to bottom of filter bed. After back washing, the three materials are mixed thoroughly through out the depth of the bed. However, at each level in the bed particles of coarse coal, medium sand and fine garnet are present.
Particles of turbidity in the influent to the mixed filter first pass through large pores and encounter the coarse media then reach smaller pores resulting from the mixing of finer media with coarser occur. Materials are removed and sorted throughout the full depth of the bed.
The vast storage capacity of the mixed media filters greatly inceases the length of filter runs.
The total surface area of the grains is much more resistant to break-through and more tolerant to increases in filter rates.
It is thus possible to achieve filter rates of 1500litres/m2/hr.
Activated Carbon filters is based on liquid to solid adsorption process which remove a solute from water via a selective solid resin. Physical adsorption involves weak Vanderwaal’s forces. Chemical adsorption involves stronger interactions (e.g ion excahange or hydrogen banding between the solid and the solute).The solids may be
Activated carbon
Activated alumina
Organoclays etc.
Adsorption can occur in fluidized, move or fixed bed as wll as in upflow pressure or gravity contact systems (in which the adsorbant is dropped through a column of water). The fixed resin bed is typically used in waste water treatment, it involves passing waste water down through a paxked column of resin.
Activated carbon is the most popular adsorbant because it is
Relatively inexpensive
Adsorbs wide range of organic and inorganic substances.
Can be made from several materials e.g wood, sawdust, fruit pits, coconut shells, coal lignite and petroleum residues.
To make activated carbon the chosen material is carbonized and then activated with hot air or steam. The extensive pore structure provides an extensive adsorption surface, which can range from 600-1100m2/g of active carbon.
In waste water treatment systems, multiple solutes often complete for adsorption sites on the activated carbon. Differences in carbon source, temperature swings and other systemspecific variances require that the pilot tests should be conducted before designing an activated carbon process.
Activated carbon is thought to remove a solute from solvent in 3 steps:-
Transfer across a liquid boundry
Sorption to the outer surface
Migration into deep pores.
Many full-scale designs incorporate two or three columns. In two column design, one column regenerates, while the other is on-line. In three-column design, two columns operate in series while the third regenerates.
Disinfection of water
None of the previous methods used for treatment of waste water either singly or in combination can be relied upon to remove the bacteria present in water especially pathogenic one. Therefore it is necessary to disinfect or kill the bacteria. The methods employed to disinfect water depends upon the disinfecting materials used for the purpose. The general requirement is
It should be harmless and unobjectionable to the consumer
It should retain a residual disinfecting effect for a long time.
Principle methods used for disinfection are
Chlorination
Ozonization
Ultraviolet ray method
Excess lime method
Iodine and bromine methods
1) Chlorination
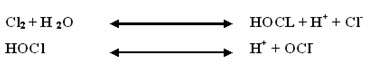
It is the application of small quantity of chlorine or chlorine compounds in water. The dose applied is generally less than 1mg/l so as to produce residual chlorine of trace quantity i.e 0.05-0.20mg/l. Chlorine hydrolysis in water to form hypochlorous acid (HoCl) which further dissociates to produce hypochlorite ion (ocl-). HoCl and oCl- together are known as free available chlorine. The reaction is as follows:
Chlorine is generally applied after all other treatments to the water has been given. This may be termed as post chlorination and is the standard treatment for all water works. There are however, other special methods of chlorination depending upon particular purpose.
i) Pre-chlorination: It is the application of chlorine preceding filteration. It reduces bacterial load on filters resulting in increased filter runs and oxidizes excessive organic mater thus removing taste and odour.
ii) Double chlorinaton: Application of chlorine at two points i.e prechlorination and to the final effluent.
Advantages are:
- Decreases in load of filters.
- Greater removal of bacteria –higher bacterial efficiency
- Greater factor of safety due to maintenance of 2 chlorinating plants.
- Control of algae and slimy growths in coagulating basins and filters.
iii) Superchlorination: It is the application of water with excess amount of chlorine i.e < 1mg/l to 2 mg/l this method is effective in destroying high concentratation of tastis and odours in water. Bacteria removal is also high.
iv) Breakpoint chlorination: It is also termed as free-residual chlorination which involves the addition of sufficient chlorine so as to oxidize a organic matter, reducing substances and free ammonia in raw water leaving behind mainly free available cl2 which possess strong disinfecting action against pathogens.
2) Ozonization
It is a highly oxidizing agent containing 3 atoms of oxygen (O3) which changes to O2 and releases nascent oxygen (O). the nascent oxygen reduces organic matter present in water without production of objectionable tastes and odours as with chlorine.
The ozone dose is 2-3mg/l to give a trace to 0.1 mg/l residual after 10 minutes contact. It is regarded as a natural means of disinfecting water having bacterial spores. However, it is costly to manufacture, it has very little residuals presents, not quite suitable for highly turbid waters.
3) Ultraviolet rays
It is an effective method of disinfecting clear water which kills all type of bacteria, cysts and spores. Water is passed over a uv lamp as the effective penetration of rays in water is only for a depth of 30cm or so.
Advantages
No taste and no odour
Presenting no danger of overdose
Disadvantages
High cost
Absence of any residual action.
Use in restricted normally to small installations like swimming pools.
4) Excess lime method
Excess lime method involves application of sufficient lime for the combined objectives of softening and disinfecting of water coliform reduction may be as high as 99%
Dose given is between 10-20mg/l
It frequently becomes necessary to remove excess lime after the process through recarbonation.
5) Iodine and Bromine methods
Their use is restricted to small water supplies such as army camps and swimming pools. Water which is muddy or colored needs to be filtered before disinfection. These are cheaply available in the form of pellets. Dosage is about 8-10mg/l. for heavily polluted water, it may be doubled only objection is the resulting medical taste.