Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Module 11. Lubricants properties, mechanism, class...
Module 12. Polymers type of polymerization, proper...
Lesson 4. Fuels and its testing
4.1 Performance Evaluation of Fuels
This section explains the principles of combustion, how fuel performance can be evaluated using the stochiometric calculation of air requirement, the concept of excess air, and the draft system of exhaust gases.
Principles of Combustion:
Combustion process
Combustion refers to the rapid oxidation of fuel accompanied by the production of heat, or heat and light. Complete combustion of a fuel is possible only in the presence of an adequate supply of oxygen. Oxygen (O2) is one of the most common elements on earth making up 20.9% of our air. Rapid fuel oxidation results in large amounts of heat. Solid or liquid fuels must be changed to a gas before they will burn. Usually heat is required to change liquids or solids into gases. Fuel gases will burn in their normal state if enough air is present. Most of the 79% of air (that is not oxygen) is nitrogen, with traces of other elements. Nitrogen is considered to be a temperature reducing diluter that must be present to obtain the oxygen required for combustion. Nitrogen reduces combustion efficiency by absorbing heat from the combustion of fuels and diluting the flue gases. This reduces the heat available for transfer through the heat exchange surfaces. It also increases the volume of combustion by-products, which then have to travel through the heat exchanger and up the stack faster to allow the introduction of additional fuel-air mixture. This nitrogen also can combine with oxygen (particularly at high flame temperatures) to produce oxides of nitrogen (NOx), which are toxic pollutants. Carbon, hydrogen and sulphur in the fuel combine with oxygen in the air to form carbon dioxide, water vapour and sulphur dioxide, releasing 8,084 kcals, 28,922 kcals and 2,224 kcals of heat respectively. Under certain conditions, carbon may also combine with oxygen to form carbon monoxide, which results in the release of a smaller quantity of heat (2,430 kcals/kg of carbon). Carbon burned to CO2 will produce more heat per unit of fuel than when CO or smoke are produced.
Three T’s of combustion
The objective of good combustion is to release all of the heat in the fuel. This is accomplished by controlling the "three T's" of combustion which are (1) Temperature high enough to ignite and maintain ignition of the fuel, (2) Turbulence or intimate mixing of the fuel and oxygen, and (3) Time, sufficient for complete combustion.
Commonly used fuels like natural gas and propane generally consist of carbon and hydrogen. Water vapour is a by-product of burning hydrogen. This removes heat from the flue gases, which would otherwise be available for more heat transfer.
Natural gas contains more hydrogen and less carbon per kg than fuel oils and as such produces more water vapour. Consequently, more heat will be carried away by exhaust while firing natural gas. Too much, or too little fuel with the available combustion air may potentially result in unburned fuel and carbon monoxide generation. A very specific amount of O2 is needed for perfect combustion and some additional (excess) air is required for ensuring complete combustion. However, too much excess air will result in heat and efficiency losses. Not all of the fuel is converted to heat and absorbed by the steam generation equipment.
Usually all of the hydrogen in the fuel is burned and most boiler fuels, allowable with today's air pollution standards, contain little or no sulphur. So the main challenge in combustion efficiency is directed toward unburned carbon (in the ash or incompletely burned gas), which forms CO instead of CO2.
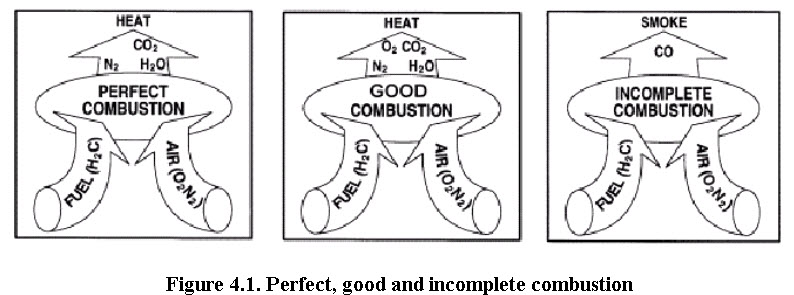
(Bureau of Energy Efficiency, 2004)
Stochiometric Calculation of Air Requirement
Calculation of stochiometric air needed for combustion of furnace oil
For combustion air is needed. The amount of air needed can be calculated using the method given below.
The first step is to determine the composition of the furnace oil. Typical specifications of furnace oil from lab analysis is given below:
If we take these analysis data, and considering a sample of 100 kg of furnace oil, then the chemical reactions are as follows:
Element Molecular Weight (kg / kg mole)
C 12
O2 32
H2 2
S 32
N2 28
CO2 44
SO2 64
H2O 18
C + O2→ CO2
H2 + 1/2O2→H2O
S + O2 →SO2
Constituents of fuel
C + O2 →CO2
12 + 32 →44
12 kg of carbon requires 32 kg of oxygen to form 44 kg of carbon dioxide therefore 1 kg of carbon requires 32/12 kg i.e 2.67 kg of oxygen
(85.9) C + (85.9 x 2.67) O2 →315.25 CO2
2H2 + O2 →2H2O
4 + 32 →36
4 kg of hydrogen requires 32 kg of oxygen to form 36 kg of water, therefore 1 kg of hydrogen requires 32/4 kg i.e. 8 kg of oxygen.
(12) H2 + (12 x 8) O2 →(12 x 9 ) H2O
S + O2 → SO2
32 + 32 →64
32 kg of sulphur requires 32 kg of oxygen to form 64 kg of sulphur dioxide, therefore 1 kg of sulphur requires 32/32 kg i.e. 1 kg of oxygen
(0.5) S + (0.5 x 1) O2 →1.0 SO2
Total oxygen required = 325.57 kg
(229.07+96+0.5)
Oxygen already present in
100 kg fuel (given) = 0.7 kg
Additional oxygen required = 325.57 – 0.7
= 324.87 kg
Therefore quantity of dry air needed = (324.87) / 0.23
(air contains 23% oxygen by weight)
= 1412.45 kg of air
Theoretical air required = (1412.45) / 100
= 14.12 kg of air / kg of fuel
Therefore, in this example, for each kg of furnace oil burnt, 14.12 kg of air is required.
4.2 Calculation of theoretical CO2 content in the flue gases
It is necessary to also calculate the CO2 content in the flue gases, which then can be used to calculate the excess air in the flue gases. A certain amount of excess air is needed for complete combustion of furnace oils. However, too much excess air points to heat losses and too little excess air points to incomplete combustion. The CO2 in flue gases can be calculated as follows:
Nitrogen in flue gas = 1412.45 – 324.87
= 1087.58 kg
Theoretical CO2% in dry flue gas by volume is calculated as below:
Moles of CO2 in flue gas = (314.97) / 44 = 7.16
Moles of N2 in flue gas = (1087.58) / 28 = 38.84
Moles of SO2 in flue gas = 1/64 = 0.016
Theoretical CO2 % by Volume = (Moles of CO2 x 100) / Total Moles (Dry)
= (7.16 x 100) / (7.16 + 38.84 + 0.016)
= 15.5%
4.3 Calculation of constituents of fuel gas with excess air
Now we know the theoretical air requirements and the theoretical CO2 content of flue gases. The next step is to measure the actual CO2 percentage in the flue gases. In the calculation below it is assumed that the measured %CO2 in the flue gas is 10%.
% Excess air = [(Theoretical CO2%/Actual CO2) – 1] x 100
= [(15.5/10 – 1)] x 100
= 55%
Theoretical air required for 100kg of fuel burnt = 1412.45 kg
Total quantity of air supply required with 55% excess air = 1412.45 x 1.55
= 2189.30 kg
Excess air quantity (actual – theoretical excess air) = 2189.30 – 1412.45
= 776.85
O2 (23%) = 776.85 x 0.23
= 178.68 kg
N2 (77%) = 776.85 – 178.68
= 598.17 kg
The final constituents of flue gas with 55% excess air for every 100 kg fuel is as follows:
CO2 = 314.97 kg
H2O = 108.00 kg
SO2 = 1 kg
O2 = 178.68 kg
N2 = 1685.75 kg (= 1087.58 in air + 598.17 in excess air)
4.4 Calculation of theoretical CO2% in dry fuel gas by volume
Now that we have the constituents by weight, we can calculate the constituents on a volume basis as follows:
Moles of CO2 in flue gas = 314.97 / 44 = 7.16
Moles of SO2 in flue gas = 1/64 = 0.016
Moles of O2 in flue gas = 178.68 / 32 = 5.58
Moles of N2 in flue gas = 1685.75 / 28 = 60.20
Theoretical CO2% by volume = (Moles of CO2 x 100) / Total moles (dry)
= (7.16 x 100) / (7.16 + 0.016 + 5.58 + 60.20)
= 10%
Theoretical O2% by volume = (5.58 x 100) / 72.956
= 7.5%
4.5 Concept of Excess Air
For optimum combustion, the real amount of combustion air must be greater than that required theoretically. Part of the stack gas consists of pure air, i.e. air that is simply heated to stack gas temperature and leaves the boiler through the stack. Chemical analysis of the gases is an objective method that helps to achieve finer air control. By measuring CO2 or O2 in flue gases (by continuous recording instruments or Orsat apparatus or some cheaper portable instruments) the excess air level and stack losses can be estimated. The excess air to be supplied depends on the type of fuel and the firing system.
A faster way to calculate the excess air is by using the figures 4.1 and 4.2, provided the percentage of CO2 or O2 in the flue gases have been measured
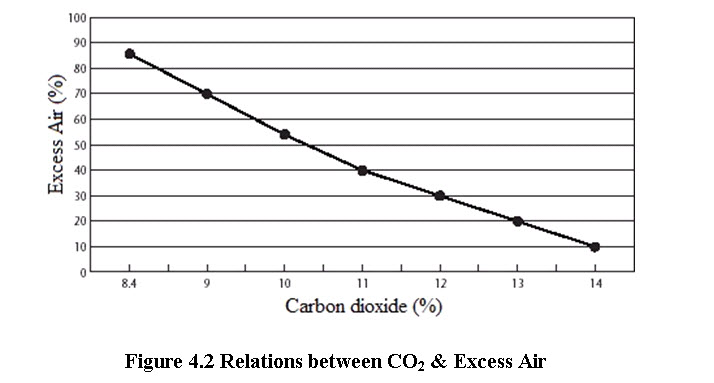
(Bureau of Energy Efficiency, 2004)
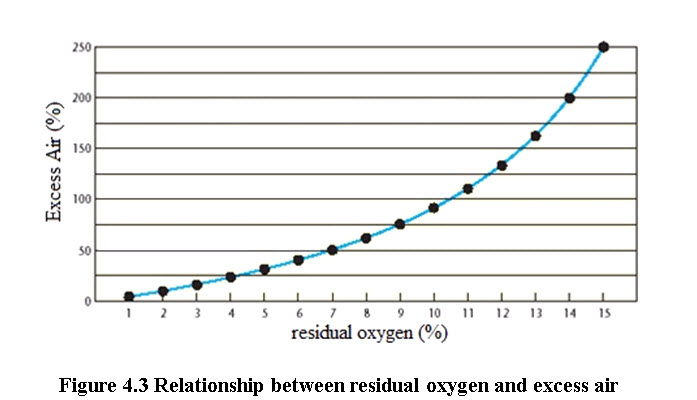
(Bureau of Energy Efficiency, 2004)
For optimum combustion of fuel oil the CO2 or O2 in flue gases should be maintained as follows:
CO2 = 14.5–15 %
O2 = 2–3 %
Draft System
The function of draft in a combustion system is to exhaust the products of combustion, i.e. flue gases, into the atmosphere. The draft can be classified into two types namely natural draft and mechanical draft.
Natural draft
Natural draft is the draft produced by a chimney alone. It is caused by the difference in weight between the column of hot gas inside the chimney and column of outside air of the same height and cross section. Being much lighter than outside air, chimney flue gas tends to rise, and the heavier outside air flows in through the ash pit to take its place. Draft is usually controlled by hand-operated dampers in the chimney and breeching connecting the boiler to the chimney. Here no fans or blowers are used. The products of combustion are discharged at such a height that it will not be a nuisance to the surrounding community.
Mechanical draft
It is draft artificially produced by fans. Three basic types of drafts that are applied are:
- Balanced draft: Forced-draft (F-D) fan (blower) pushes air into the furnace and an
induced draft (I-D) fan draws gases into the chimney thereby providing draft to remove the gases from the boiler. Here the pressure is maintained between 0.05 to 0.10 in. Of water gauge below atmospheric pressure in the case of boilers and slightly positive for reheating and heat treatment furnaces.
- Induced draft: An induced-draft fan draws enough draft for flow into the furnace, causing the products of combustion to discharge to atmosphere. Here the furnace is kept at a slight negative pressure below the atmospheric pressure so that combustion air flows through the system.
- Forced draft: The Forced draft system uses a fan to deliver the air to the furnace, forcing combustion products to flow through the unit and up the stack.
Energy Efficiency Opportunities
This section includes energy efficiency opportunities in Fuel Combustion:
Pre-heating of the Combustion Oil
The viscosity of furnace oil and LSHS (Low Sulphur Heavy Stock) increases with decreasing temperature, which makes it difficult to pump the oil. At low ambient temperatures (below 25 0C), furnace oil cannot be pumped easily. To avoid this, preheating of oil is accomplished in two ways:
The entire tank may be preheated. In this form of bulk heating, steam coils are placed at the bottom of the tank, which is fully insulated;
The oil can be heated as it flows out with an outflow heater. To reduce steam
requirements, it is advisable to insulate tanks where bulk heating is used.
Bulk heating may be necessary if flow rates are high enough to make outflow heaters of adequate capacity impractical, or when a fuel such as LSHS is used. In the case of outflow heating, only the oil, which leaves the tank, is heated to the pumping temperature. The outflow heater is essentially a heat exchanger with steam or electricity as the heating medium.
Temperature control of Combustion Oil
Thermostatic temperature control of the oil is necessary to prevent overheating, especially when oil flow is reduced or stopped. This is particularly important for electric heaters, since oil may get carbonized when there is no flow and the heater is on. Thermostats should be provided at a region where the oil flows freely into the suction pipe. The temperature at which oil can readily be pumped depends on the grade of oil being handled. Oil should never be stored at a temperature above that necessary for pumping as this leads to higher energy consumption.
Preparation of Solid Fuels
Sizing of coal
Proper coal sizing is one of the key measures to ensure efficient combustion. Proper coal sizing, with specific relevance to the type of firing system, helps towards even burning, reduced ash losses and better combustion efficiency. Coal is reduced in size by crushing and pulverizing. Pre-crushed coal can be economical for smaller units, especially stoker-fired units. In a coal handling system, crushing is limited to an upper size of 6 or 4mm. The devices most commonly used for crushing are the rotary breaker, the roll crusher and the hammer mill. It is necessary to screen the coal before crushing, so that only oversized coal is fed to the crusher. This helps to reduce power consumption in the crusher. Recommended practices in coal crushing are:
Incorporation of a screen to separate fines and small particles to avoid extra fine generation in crushing.
Incorporation of a magnetic separator to separate iron pieces in coal, which may damage the crusher.
Conditioning of coal
The fines in coal present problems in combustion on account of segregation effects. Segregation of fines from larger coal pieces can be reduced to a great extent by conditioning coal with water. Water helps fine particles to stick to the bigger lumps due to surface tension of the moisture, thus stopping fines from falling through grate bars or being carried away by the furnace draft. While tempering the coal, care should be taken to ensure that moisture addition is uniform and preferably done in a moving or falling stream of coal. If the percentage of fines in the coal is very high, wetting of coal can decrease the percentage of un burnt carbon and the excess air level required for combustion. The table below shows the extent of wetting, depending on the percentage of fines in coal.
Blending of coal
In the case of coal containing excessive fines, it is advisable to blend the predominantly lumped coal with lot of coal that contains excessive fines. Coal blending may thus help to limit the extent of fines in coal being fired to not more than 25%. Blending of different qualities of coal may also help to supply a uniform coal feed to the boiler.
4.4 Combustion Controls
Combustion controls assist the burner in regulation of fuel supply, air supply, (fuel to air ratio), and removal of gases of combustion to achieve optimum boiler efficiency. The amount of fuel supplied to the burner must be in proportion to the steam pressure and the quantity of steam required. The combustion controls are also necessary as safety device to ensure that the boiler operates safely.
Various types of combustion controls in use are:
On/Off control: The simplest control, ON/OFF control means that either the burner is firing at full rate or it is OFF. This type of control is limited to small boilers.
High/low/off control: Slightly more complex is HIGH/LOW/OFF system where the burner has two firing rates. The burner operates at slower firing rate and then switches to full firing as needed. Burners can also revert to the low firing position at reduced load. This control is fitted to medium sized boilers.
Modulating control: The modulating control operates on the principle of matching the steam pressure demand by altering the firing rate over the entire operating range of the boiler. Modulating motors use conventional mechanical linkage or electric valves to regulate the primary air, secondary air, and fuel supplied to the burner.
Full modulation means that boiler keeps firing, and fuel and air are carefully matched over the whole firing range to maximize thermal efficiency.
Biomass
Importance of Biomass
Prior to the industrial revolution, wood and agricultural residues are the primary source of energy for sectors like industry, transport and domestic. The transition took place from wood to coal and to oil and treated wood and agricultural residues as old fuels. Today, many fossil fuel based technologies have reached more advanced form of technological maturity, dependability access and efficiencies, incomparable to past few decades, thus only leaving more scope for conservation and search for new alternatives than changing the pattern of consumption and the domestic oil reserves are insufficient forcing import of oil and related products leading to pollution, global warming etc. The concerted efforts are required to identify new resources to put back the old fuel resources for meeting the requirements on large scale for local area energy demands. This helps in reducing the import of oil and naturally economics will recover. This kind of reversals is quite possible since the time is ripe and ideal to focus on utilization of biomass as a source of energy, food and fodder. Among various renewable energy sources, biomass offers a wide range of products to meet the present days’ challenges. Unlike other forms of renewable energy sources, biomass offers a wide variety of bi-products similar to the fuels presently derived from fossils for other applications, where as the residues still offer their energy potential. So biomass is the best and dependable.
A practicable long term strategy helps us to store large quantities of energy in the form of biomass, whereas large investments are required to store solar energy or wind energy in batteries. However, such a large amount of solar energy can be stored in the form of biomass by the process called photosynthesis.
Exploiting Biomass as a Fuel
Over selling biomass as a source of fuel, may force us to utilize indiscriminately leading to an expensive concept of whole plant utilization. Most of the earth’s fertile soil is made of biomass due to death and decay. This allows and supports the standing of crops and trees. To provide macro and micro-nutrients and organic constituents required for an active soil, the minimum required biomass must be left out returnable to the soil. If this requirement is not met, the starving soils will give no biomass to the generation to come. Hence, there is a need to postulate some restrictions based on benchmark studies and to recommend the quantities of biomass to be left for soils without fail. Hopefully, a regularity mechanism to monitor soil fertility and biomass as energy option helps sustainability.
Scope of Biomass Feed Stocks
Plant stores the solar energy in the form of fixed carbon achieved by the biochemical process called photosynthesis. This biochemical potential is stored by the plants in various forms at different stages of its growth. Based on the nature of these forms of availability, feed stocks can be classified as follows.
1 Woody: The wood can be made available from forests and energy plantations. Wood have high density is ideal for direct combustion, gasification and also for the production of ethanol and oils.
2 Non-woody: Small branches of trees, annually available agricultural residues like cotton stalk, maize, corn, pulses stalk, bagasse, tobacco etc terrestrial weeds like prosophis, lantana, parthenium and others. Though bulk densities are low, well suited for gasification, direct combustion and for production of ethanol.
3 Process residues: The process residues like rice husk, coconut shells saw dust, coir pith, groundnut shells, cashew nut shells, etc. The bulk densities are reasonable with advantage of suitable material for gasification and direct burning.
4 Aquatic plants: Water hyacinth, lemma, algae, marsh reeds, sea weeds etc. subjected to anaerobic fermentation for biogas. Direct combustion and gasification have limited application due to high moisture content.
5 Plants oils and fluids: Agricultural crops like groundnut, soybean, sunflower, sesame etc. yield vegetable oils which are suitable for diesel substitution also. However, these oils are basically edible in nature. The residues viz., oil cakes offer the opportunity for producing appreciable amounts of biogas and the slurry is a good soil conditioner and manure. For eg. oils from Neem, pongamia, mahua, sal and the like produce oils, which are basically non-edible in nature. As on today, these oils are mostly used in sectors like pharmaceuticals, lubricants, dying and others. There are above 120 species classified under non-edible grade oil category both tree-born and crop based. These oils have very high value for substitution for diesel.
Biomass availability
The biomass available annually, was put to use only to an extent of 50% and remaining is wasted or lost. Dependence on forests for wood should be discouraged and to provide incentives of utilization for excess available crop residues for judicial application for thermal and power application.
The following various seven options available for the use of biomass
Direct combustion as cooking fuel or for the industrial usage
Charcoal production and utilization
Thermal power generation
Production of producer gas
Biogas production
Alcohol production and its utilization
Compaction of solid biomass in the form of briquettes and briquettes utilization
Advantages and Disadvantages
Biomass has both advantages as well as disadvantages in being used as an energy source which are enumerated below.
i. The biomass provides an effective low sulphur fuel.
ii. It provides an inexpensive and readily available source of energy, and
iii. Processing biomass materials for fuel reduce the environmental hazard.
The major problems or difficulties in utilizing biomass for solar energy conversion as well as a renewable source of energy are:
i. The relatively small percentage (less than 0.1%) of light energy is converted into biomass by plants.
ii. The relatively sparse and low concentration of biomass per unit area of land and water
iii. The scarcity of additional land suitable for growing plants.
iv. Scattered and seasonal availability of biomass
v. Their large volume and low bulk density associated with high moisture content that makes their collection and transport expensive and energy conversion relatively inefficient.
vi. The very abrasive nature due to silica cellulose structure causing high wear and tear in grinding machinery and fragile and porous nature of some agro industrial residues like rice husk makes them difficult to be stored in outdoor piles which become vulnerable to fire hazard and be air borne by the wind.
These methods are based on thermal, chemical and enzymatic conversion processes. In dry process, material is transformed under high temperature In wet process, Biological processes such as fermentation are involved.
Physical Processes / Mechanical Methods
This method is used to increase the bulk density of biomass material for easy transportation and storage and involves the following processes.
I. Briquetting: The briquetting is the process of making small size compressed block to get more surface area per unit weight of biomass by adding suitable binder. It includes the following process.
a) Moisture removal: The process involves the removal of moisture from contents to break down its elasticity to reduce its volume sufficiently.
b) Densification: The product is carried out at high pressure and temperature 1800C to get more homogeneous product after moisture is removed.
II. Pelletization: Fuel pellets or refused derived fuel (RDF) are small cubes mode from the solid waste/garbage and are used as a fuel for boilers to produce steam or electricity. Pelletization of the wood is carried out by compressing it in the forms of rods of small diameter 5-12 mm in the
extruder after removal of moisture 7-10%.
III. Size Reduction: Making small pieces by using shredding machines or Hammer mills.
Different types of dry processes
1. Combustion: Direct combustion is a complete oxidation process where liberation of heat is the primary objective. Burning of any substance in excess air whether it be solid, liquid or gas is termed as combustion. In combustion, a fuel is oxidized evolving heat and often light
C+O2 → CO2 + Heat
The combustion of solid fuel occurs in stages. i.e. Actual combustion of organic residues is not a single process but a combination of processes occurring simultaneously.
Three overlapping phases can be identified as
1. Initial phase: Evaporation of moisture
2. Volatilization and burning of volatiles: Dry matter absorbs heat, drives off volatile gases by thermal decomposition of the fuel and burning of volatiles in the air occurs.
3. Final phase: Fixed carbon burns
At usual combustion temperature, the burning of hot solid residue is controlled by rate at which oxygen of the air diffuses to its surface. If the residue is cooled by the radiation of heat, combustion ceases.
Eg: Open chulas thermal efficiency : 3 – 4 %
Improved chulas thermal efficiency : 10 – 14%
Consumption of the fuel : 0.5 – 1 Kg /per person
Use: To produce steam for process use and for electricity.
2. Pyrolysis: Pyrolysis is defined as the destructive distillation of organic material heated to more than 2000C in the absence of air/oxygen for several hours to produce combustible gases (H2, CO, CH4), other hydrocarbons, CO2 and N2, solid char, liquid tar and organic liquids. It is a non oxidative thermal process that results in gases, liquids and char. It is thermal degradation of cellulose. In practice, many processes allow a restricted admission of air for partial combustion to achieve the temperature required for pyrolysis. The temperature of pyrolysis, composition of biomass (C, H, N, O, S), retention period and heating rate in pyrolyser etc determine the nature and quantum of these products. The slow rate of heating, low temperature and adequate retention time tend to give high yields of char.
3. Gasification: It is a chemical change involve several chemical reactions which occur simultaneously at varying rates. The dry process is combustion of biomass in a controlled atmosphere. This is a gasification process involving the burning of biomass with a limited air supply at temperature above 11000C. The typical product is a mixture of CO + H2 i.e. producer gas which is 1/10th of the petroleum gas. Useful for cooking, heating and electricity generation.
4. Liquification: The dry process of biomass conversion is hydro carbonization or liquification, which combines high temperature and pressure to produce oil or gas. These Fuels are same as that of petroleum and natural gas. But the operating cost and energy expenditure higher