Module 7. Mass transfer
Lesson 31
PROBLEMS BASED ON HEAT AND MASS TRANSFER
Q. 1. Evaluate the diffusion coefficient of CO2 in air at 20°C and atmospheric pressure.
The following data is given:
|
|
|
|
|
CO2 |
3.996 |
190 |
|
Air |
3.617 |
97 |
Π = 1.044
Solution:
![]()
![]()
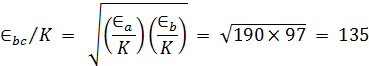
T = 20+273 = 293°K
P = 1 atm
![]()
![]()
Π = 1.044
![]()
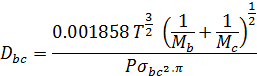
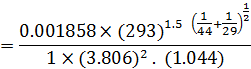
= 0.147 cm2/ sec.
Q. 2. Estimate the diffusion rate of water from the bottom of a test tube 1.5 cm in diameter and 15 cm long into dry atmospheric air at 25°C.
Assume D = 0.256 cm2/s
Solution:
Air will be saturated at the bottom of the test tube at x = 0 then the partial pressure of water vapour at x = 0 will be saturation pressure of water vapour at 25°C.
![]()
at x =L =15cm (length of the tube)
![]()
At the top, air does not contain any water vapour
![]() Pw2
= 0
Pw2
= 0
![]() Pa2
= Pt = 1.0303.
Pa2
= Pt = 1.0303.
Now using the following equation
![]()
![]()
D= 0.256 × 0.36 m2/hr, T = 25+273 Mw =18
Pt = 1.0303 ata= 1.0303 ×104 kg/m2 , x1 = 0 , x2 = 0.15 m
G = 848
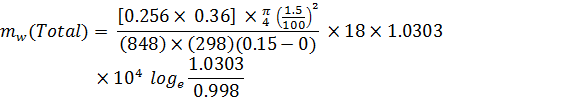
= 1.086 × 10-6 kg/hr.
Q. 3. Calculate the rate of diffusion of water vapor from a pool of water at the bottom of a well 6 metre in height to dry air flowing over the top of the well. Assume the entire system is at 25°C and 1 atm. If the well diameter is 3 meters, then find out the total weight of water diffused per hour from the surface of the water in the well.
Assume D = 0.256 cm2/s
Solution:
D = 0.256 x 0.36 m2/hr, x1 = 0, x2 = 6 m
Pa1 = Pt – Pw1 =1.0303 – 0.0323 = 0.998
Pa2 = Pt – Pw2 =1.0303 – 0.00 = 1.0303
A = 1 m2, Mw = 18, G = 848, T = 298.
Using the following equation
![]()
![]()
![]()
= 1.125 ×10-4 kg/m2-hr.
Total mass flow = mass flow rate × well surface area
= 1.125 × 10-4
× π/4 (3)2 =
7.94 × 10-4
kg/hr.