Module 3. First law of thermodynamics
Lesson 5
NON-FLOW AND FLOW PROCESSES
5.1 Non-Flow Processes
5.1.1 Adiabatic process
Adiabatic process is one in which there is no exchange of heat between system and surroundings
i.e. Q= 0.
Applying first law
of thermodynamics to this process
0
= δW
+ dU or δW = -dU
So, dU = −δW = −P.dV
(Eq. 5.1)
Or in specific terms du = - δw = - P.dv
![]()
Also from the
definition of enthalpy, the change in specific enthalpy is
![]() (Eq. 5.2)
(Eq. 5.2)
Putting eq 5.1 into eq 5.2
dh = vdP
Thus in an
adiabatic process
![]() (Eq. 5.3)
(Eq. 5.3)
And dh = CpdT = v.dP
(Eq. 5.4)
Dividing eq 5.4 by eq 5.3
![]()
or
![]()
or
![]()
Integrating on both sides
![]()
or
![]()
or
![]() =Constant
=Constant
or
![]()
or
![]() (Eq. 5.5)
(Eq. 5.5)
This represents a reversible adiabatic process: 1-2
From gas law
![]()
or
![]() (Eq. 5.6)
(Eq. 5.6)
or ![]()
Putting the value of P1/P2 from equation eq 5.5
![]()
![]()
![]()
![]()
![]()
![]()
![]()
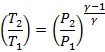
Thus the overall relation between initial and final properties (Pressure,
volume and temperature) in an adiabatic process 1-2 is
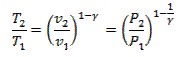 (Eq. 5.7)
(Eq. 5.7)
Work exchange
We know that work exchange during a non-flow process is given as
W= ![]()
Thus work during non-flow reversible adiabatic process 1-2 is
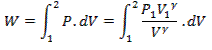 from (Eq. 5.5)
from (Eq. 5.5)
![]()

![]()
![]()
![]()
Specific work i.e. work per unit mass will be
![]() (Eq. 5.8)
(Eq. 5.8)
If work exchange comes as positive that means gas is doing work on its
surrounding during the process and if it comes as negative, the work is being
done on the gas.
Also in the reversible adiabatic process 1-2, Q = 0 (no heat exchange) so
as per first law of thermodynamics:
∆U = -W
Or
![]() (Eq. 5.9)
(Eq. 5.9)
5.1.2 Polytropic process
In real practice it is found that an ideal gas while undergoing a non-flow
process which may be any one or combination of two of the heating / cooling and
compression / expansion processes, follows the law.
PVn =
Constant
Where n is known as index of compression or expansion. (Fig. 5.1)
It is a general form of any non-flow process and the value of n decides
the particular type of process. For example
·
If n = 0 ↔ then PV0 = constant Or P =
constant
↔ constant pressure process.
·
If n = ∞ then PV∞ = constant
or P1/∞.V = constant or P0V
= constant or V = constant
↔ constant volume process
·
If n = 1 then PV1 = PV = Constant.
Mixing it with ideal gas law ![]()
If PV = Constant, then T = constant
⟷ constant
temperature process
·
If n = γ then P![]() = Constant
= Constant
↔ adiabatic process
·
If n has any other value except 0, 1, γ and ∞
↔ polytrophic process
Depending on the value of n, all these processes can be represented on the
PV- diagram in Fig. 5.2
as follows.
Combining the polytrophic process law PVn = Constant with the
ideal gas law![]()
![]()
the relation between initial and final properties (pressure, volume and
temperature) in a polytropic process1-2 can be derived as
 (Eq. 5.10)
(Eq. 5.10)
5.1.2.1 Heat / work exchange
The difference in mathematical law governing an adiabatic and polytropic
process is only of γ and n. So work exchange in a polytropic process
can also be similarly derived as in case of adiabatic process and it will be
![]()
And specific work,
![]() (Eq. 5.11)
(Eq. 5.11)
Applying first law of thermodynamics to the polytropic process
1-2
Q = W
+ ∆U
![]()
![]()
![]()
![]()
![]()
![]() (Eq. 5.12)
(Eq. 5.12)
5.2 Flow Processes
The processes which happen in an open system are called flow processes. As
already studied, an open thermodynamic system is one in which there is not a
fixed quantity or mass of gas/vapours but the working substance continuously
flow in or out of the system. When this flowing substance or fluid exchange
heat & work energy with the surrounding, the process is called flow
process. Examples of this type of system are IC engines, centrifugal pump,
steam turbines, water turbines etc. These flow systems may further be of the
two types as steady flow system and unsteady flow system as discussed below:
5.2.1 Steady flow systems
The system through which the mass flow rate is constant i.e.
Mass Input = Mass Output
is a steady flow system. The state of working substance at
any point in this system remains constant. Examples: Most actual thermodynamic
equipment work as steady flow system under steady state conditions. Examples
are IC engine, Compressors, steam turbines etc.
5.2.1.1 Analysis of steady flow system
Under steady state, Total Inlet Energy = Total Outlet
Energy
Now Total Energy = I.E. + F.E + K.E + P.E
Where
I.E. = Internal Energy = mu
F.E. = Flow Energy = mPv
K.E. = Kinetic Energy = ![]() mV2
mV2
P.E. = Potential Energy = mgZ
So, ![]()
![]()
In steady flow system the rate of mass flow is constant.
i.e. Mass Input =
Mass output
Or m1 = m2

Fig. 5.3 Open system
Also let the height of working substance at any point in the
system remains constant. i.e.
z1 = z2.
And the kinetic energy
is usually so small w.r.t. heat, work & enthalpy term, it can also be
neglected.
Also taking U1 + P1V1 = h1
(enthalpy)
H1 + Q = H2 + W
Or Q = dH +
W
(Eq. 5.13)
Or dH = δQ
δW
Or dU + P.dV +
V.dP = dU + P.dV−dW
Or V.dP = −dW
So work done in a flow process is given as ![]() (Eq. 5.14)
(Eq. 5.14)
-ve sign indicates that work is +ve during expansion i.e.
when pressure decreases or dP is negative. And when during compression dP
is positive, work will be negative.
5.2.2 Un-steady flow system
The open system through which the mass flow rate of working
substance is not constant is un-steady flow system. Here the study of unsteady
flow system is beyond the scope of syllabus.
5.3 Internal Energy and Enthalpy of an Ideal Gas
We know that internal energy is the function of temperature
only i.e. U = f (t)
To find that function use the relation for sp. heat at
constant volume
![]()
As u is a function of temp only and ∂u/∂t is same for any
pressure or volume and either one of them vary or remain constant.
Thus
![]() (Eq. 5.15)
(Eq. 5.15)
Thus the change in internal energy of an ideal gas for a
particular change in temperature is always the same, whatever the process may
be. For an ideal gas
sp enthalpy, h = u + Pv
= u +RT (for
an ideal gas)
= f1 (t) + f2 (t)
So, specific enthalpy of an ideal gas is also a function of temperature
only i.e.
h = f (t)
To find this function use the relation for specific heat at constant
pressure.
![]()
As the enthalpy is a function of temperature only for an ideal gas i.e. the
change in enthalpy for a particular change in temperature is always the same
irrespective of the type of process
So
![]() (Eq. 5.16)
(Eq. 5.16)
