Module 7. Butter-making process
Lesson 18
PROCESS OUTLINES OF BUTTER MAKING
18.1
Introduction
Butter,
a fat rich dairy product obtained by churning cream and working the granules
thus obtained into a compact mass, has been a staple item of diet in many
countries of the world. Up to the middle of the nineteenth century, manufacture
of this product was mainly confined to the farm on cottage scales. It was only
after the development of centrifugal cream separator in 1879, fat testing
methods by Babcock (1890) and Gerber (1892) together with introduction of
artificial refrigeration and pasteurization around 1980, the industrial
production of butter developed rapidly. Prior to 1970 most of the world’s
butter was manufactured by batch-process. However, since World War-II,
continuous processes have been introduced to achieve increased manufacturing
efficiencies. Regardless of manufacturing method employed, the essential
feature of churning evolves destabilization of cream emulsion by means of
mechanical agitation.
Butter
and other fat spreads can be characterized by the type of emulsion. In milk or
cream, fat is dispersed in the continuous phase of serum while in butter, there
is a reversal of phase i.e. fat becomes the continuous phase with serum
dispersed in it. This phase reversal is carried out by churning cream in butter
churns.
Steps
involved in the conventional process of butter making which comprises
preparation of cream and churning and working are given in Figs. 18.1 and 18.2
and described below.
18.2 Preparation of Cream
Commercial butter can be produced from both sweets as well as cultured cream. Very little cultured butter is produced in India and U.S.A., although in Europe and Canada, cultured butter is an important product. However, most creamery prefer to produce butter from sweet cream as it result in sweet butter milk which has better economic value than sour butter-milk that results when sour/cultured cream is churned.
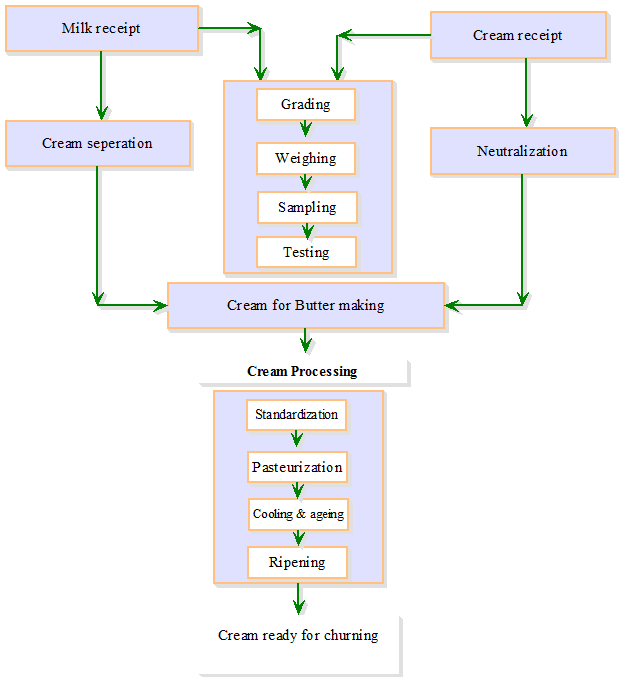
Fig. 18.1 Flow diagram of cream preparation for butter manufacturing
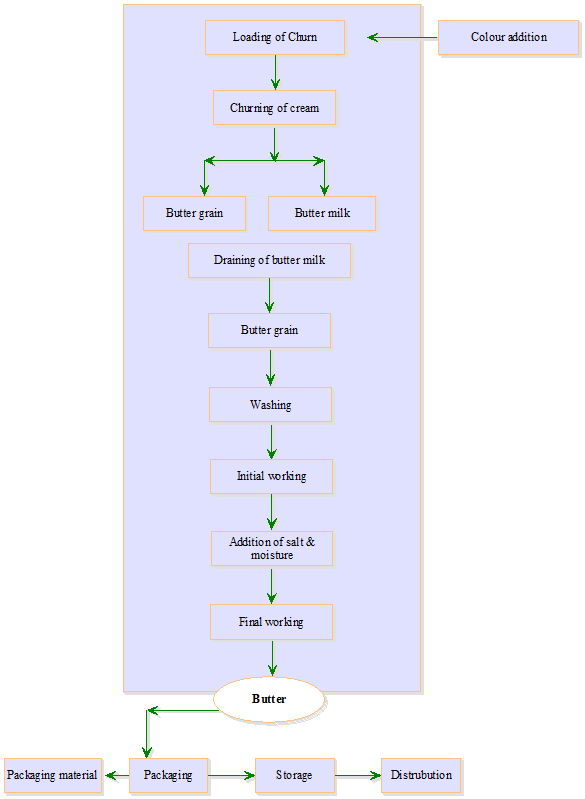
Fig. 18.2 Flow Diagram of Butter Manufacturing
18.2.1. Neutralization of cream
Sour cream must be neutralized to make butter of good keeping quality. It is under stood that by neutralization of cream acidity of cream is reduced. Churning of High acid cream may cause high fat loss which can be prevented by neutralization. In pasteurization of sour cream, the casein curdles, by entrapping fat globules, as the bulk of curd goes in butter milk, causing high fat loss.
18.2.1.1 Objectives of neutralization
- The objectives of
neutralization are to reduce the acidity in cream to a point (0.14 -0.16%)
which permits pasteurization without risk of curdling, to produce butter
which keeps well in cold storage
- To avoid excess loss of fat
which result from the churning cream i.e
excessively sour.
- To guard against undesirable
flavors which may result when a cream of high acid which is subjected for
pasteurization at higher temperatures.
- To improve the keeping quality
of butter from high acid cream. Salted-acid-butter develops a fish flavor
during commercial storage at -23 to -29°C.
18.2.1.2 Theoretical basis of cream neutralization
Soda neutralizer
NaHCO3 + CH3 CHOHCOOH----- CH3 CHOHCOONa +H2O + CO2
84 90
NF=84/90=0.933
Na2CO3 + CH3 CHOHCOOH----- CH3 CHOHCOONa +H2O + CO2
106 90
NF= 106/180=0.58
NaOH + CH3 CHOHCOOH----- CH3 CHOHCOONa +H2O
40 90
NF=40/90=0.444
Lime neutralizers
Ca (OH) 2 + 2 CH3 CHOHCOOH----- (CH3 CHOHCOO) 2Ca + 2H2O
74 90×2=180
NF= 74/180=0.411
Mg (OH)2 + 2 CH3 CHOHCOOH----- (CH3 CHOHCOO) 2Mg + 2H2O
58 180
NF= 58/180=0.322
NF = Neutralization factor
The quantitative relationship between amount of lactic acid present in solution and amount of pure neutralizer required to give exact neutralization is fixed and definite amount of CO2 is driven off by air.
Ex: 90g of lactic acid requires 84g NaHCO3 (or) 106g Na2CO3 (or) 40g of NaOH for neutralization.
18.2.1.3 Expression of acidity in cream
Acidity in cream is present chiefly in the serum portion and not in fat. The acidity in cream as a whole known as cream acidity (C.A.) while the acidity per cent found in serum is known as cream serum acidity (C.S.A). Cream serum acidity is more reliable than cream acidity.
There is a relationship between CA & C.SA as below:
CA % = Serum % in cream
CSA% 100
18.2.1.4 Factors affecting neutralization
Accurate neutralization of sour cream is important to get a desired quality product. Neutralization is influenced by several factors such as:
i. Accuracy in sampling.
ii. Accuracy in testing.
iii. Accuracy in estimation of amounts of cream and neutralizer.
iv. Careful weighing the quantity of neutralizer.
v. Thorough mixing of neutralizer in cream prior to pasteurization.
18.2.1.5
Method of neutralization of cream
There are five essential steps to follow for cream neutralization. These are:
1. Adoption of definite standard of churning acidity
2. Correct estimation of acidity
3. Calculating the amount of neutralizer to be added
4. Adding neutralizer in the correct manner
5. Checking results by re-testing acidity
Adoption of a definite standard of churning acidity
Acidity of cream at churning time controls the flavour and keeping quality of the butter. Therefore, it is important to decide that at what acidity the cream shall be churned.
Churning acidity should be kept upto that maximum acidity where freedom from chemical deterioration of butter (fishy flavour) with age can be ensured. For cream of average richness (about 30%), fishy flavor can be prevented by keeping the churning acidity to 0.3% maximum.
The safe maximum limit of churning acidity varies with the richness of the cream. Since the acidity of cream is chiefly contained in the cream serum, cream serum acidity adjustment would give better results.
![]()
e.g. 30% fat cream, cream acidity 0.25%
![]()
= 0.357%
Usually serum acidity is kept at 0.35% to achieve best keeping quality of butter.
Ex.1 cream test 20% fat, serum acidity 0.35% is desired what should be the acid test of the cream?
% cream serum: 100-20 = 80
% serum acidity = 0.35
% acid in cream = % cream serum × % serum acidity
= 0.80 × 0.35
= 0.28%
Correct Estimation of Acidity
Representative sample should be taken. Weight of cream should be accurately measured as the final amount of neutralizer will be dependent on the weight of cream.
Effect of CO2 on acidity should be taken care especially in high acid cream (acidly > 0.65%)
Calculating the
amount of neutralizer to be added
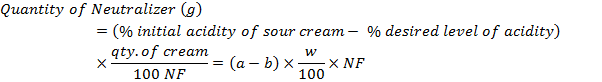
N. F. for
Sodium bicarbonate NaHCO3 1.1
Sodium bicarbonate Na2CO3 1.7
Calcium hydroxide Ca(OH)2 2.43
Magnesium hydroxide Mg(OH)2 3.1
Sodium hydroxide NaOH 2.25
Adding neutralizer in the correct manner
Neutralizer should be dissolved or emulsified in clean water, diluted to approx. 20 times its weight with water; the solution must be distributed quickly & uniformly throughout the entire batch of cream and mixed thoroughly with cream. For efficient mixing, neutralizer is usually sprayed onto the surface of well agitated cream. While the neutralizer is added, the cream should be agitated vigorously and continuously. Agitation of cream is preferable for 5-10 min after neutralization. Temperature at the time of neutralization should be 30oC. (High enough for smooth consistency & low enough to prevent abnormal heat curdling of the sour cream)
The above precautions are essential, if efficiency of neutralization, protection of butter against neutralizer flavour, oily metallic flavour and mealy body, avoidance of pasteurizing difficulties and prevention of excessive fat losses in the buttermilk are to be assured.
Checking Results of Neutralization by Re-testing for Acidity
Acidity should not be checked immediately after neutralization because of the following reasons:
- In case of lime and Magnesia
neutralizers, the neutralizing action is slow. It completes after
pasteurization & cooling.
- In case of soda neutralizers,
CO2 is liberated and this reacts acid
toward the phenolphthalein indicator. After pasteurization, expulsion of
CO2 is largely accomplished. Therefore, testing acidity after
pasteurization would give correct results.
18.2.1.6 Role of carbon dioxide in neutralization of cream with sodium bicarbonate
Fresh cream always contains some dissolved carbon dioxide (as carbonic acid) which reacts with sodium hydroxide during titration and shows a higher acidity test. But the carbon dioxide does not react with sodium bicarbonate neutralizer and consequently over neutralization results.
18.2.1.7 Double neutralization with lime and soda
The objectives of double neutralization are:
1. To avoid the intense effect on flavor of a large amount of any one neutralizer with high-acid cream.
2. To avoid production of excessive carbon dioxide by the use of sodium bicarbonate with high-acid cream
For this purpose the cream is first neutralized with lime neutralizer and brings the cream acidity down to 0.3-0.4 percent. Next use soda neutralizer to bring the cream acidity down to the desired level.
18.2.1.8 Neutralizing Precautions
In order to secure the desired results, i.e., accurate acid reduction, absence of objectionable neutralizer flavor and of excessive fat losses, make sure of the correctness of weight of cream and acid test. Do not heat the sour cream above 85 to 90°F, before neutralization, use the correct amount of neutralizer in properly diluted form, distribute it evenly over the cream, and continue agitation of the neutralized cream for 5 to 10 minutes before starting to pasteurize.
18.2.1.9 Type of Neutralizers
Neutralizers in order to accomplish the purpose, for which they are used in the creamery, must have alkaline properties. They must be alkalis, alkaline earths or their substances. An alkali is a substance that has the property of neutralizing acids, forming salts with them. The neutralizers used for reducing acidity in cream belong to either one or the other of two groups namely.
· Lime Neutralizers
· Soda Neutralizers
Lime
neutralizers
The principal constituent of the majority of lime neutralizers is calcium. Many of the lime neutralizer’s available for cream neutralization also contain some magnesium. The various commercial lime neutralizers differ from one another chiefly with respect to the proportion of calcium and magnesium they contain. They are conveniently placed in three groups, as follows:
a. Low magnesium limes: Containing 5% or less of magnesium. A well Known brand of creamery lime belonging to this group is peerless lime.
b. Medium magnesium limes: Containing about 30-35% magnesium. To this group belong such brands ad Kelly Island lime, Neutra-Lac and Neutra-Lime.
c. High magnesium limes: Containing about 45 to 55% magnesium. All wood lime is an outstanding representative of this group. All magnesium limes in the form of magnesium oxide and magnesium carbonate are also available. They are artificially prepared limes and demand a higher price than the natural limes. Their effect on the flavor of cream and butter however is outstandingly favorable.
Calcium carbonate - low solubility, low alkalinity unsuitable for cream neutralization and action is very slow.
In general the medium and high magnesium limes react somewhat more satisfactorily in the cream than the low magnesium limes. The higher the magnesium oxide content of lime the greater is its alkalinity and its neutralizing strength.
Soda neutralizers
Soda neutralizers commonly used in the creamery are:
1. Bicarbonate of soda or baking soda
2. Sodium carbonate or soda ash
3. Mixtures off baking soda and soda ash, such as Sodium sesquicarbonate, Neutralene and Wyandotte.
18.2.1.10 Comparison between lime and soda neutralizers
1. Purity: Soda Neutralizers will have less than 0.1 impurities whereas lime neutralizers will have sand and clay.
2. Solubility: Sodium Neutralizers are completely soluble whereas lime neutralizers are slightly soluble in waste.
3. Action: Sodium Neutralizers act quickly. Lime Neutralizers react slowly.
4. Action on casein: Soda neutralizers will have solvent and softening action and assist in minimizing clogging during processing. Lime neutralizers tend to granulate (or) precipitate casein which results in bitter lime flavor on pasteurization.
5. Acid reaction: Soda Neutralizers act on serum acidity first. In lime neutralizers the calcium has natural affinity towards casein, lime particles attach themselves mechanically to casein and it is not completely available for neutralizing the lactic acid. The neutralizing capacity of lime neutralizer is 80-85%.
6. Foaming effect: Soda neutralizers will produce violent foam when there is high acidity NaHCO3 has more effect than Na2CO3. Lime neutralizers do not create foam.
7. Neutralizing strength: Soda neutralizers are weaker alkalis than lime neutralizers about twice as many kilograms of soda neutralizers are required to neutralize given amount of acid as compared to lime neutralizers.
8. Material cost: Lime neutralizers are cheaper than soda neutralizers.
9. Effect on texture of butter: In case of high acid cream especially neutralizes to a low point there is a tendency to butter from lime neutralizer cream will give less smooth texture than soda neutralizers.
10. Effect on flavor of butter: Soda neutralizers produce soapy type of flavor. Lime neutralizers’ produces course lime flavor. In over all the butter will give neutralizer flavors.
18.2.2 Standardization of cream
It refers to adjustment of fat to desired level. It is done by adding calculated quantity of skim milk or butter milk. Desired level of fat in cream for butter making is 33 to 40 per cent. Standardization to both higher and lower level leads to higher fat loss in butter milk. Reduction of fat by adding water should be avoided as it interferes ripening of cream and also results in butter with ‘flat’ or ‘washed off’ flavour.
18.2.3 Pasteurization of cream
It refers to adjustment every particle of cream to a temperature not less that 71°C and holding it at that temperature for at least 20 min or any suitable temperature-time combination using properly operated equipments. The main objectives of pasteurization are: (i) it destroys pathogenic microorganisms in cream so as to make it, and the resultant butter, safe for human consumption. (ii) It also destroys bacteria, yeast, mould, enzymes and other biochemical agents that may lower keeping quality. (iii) It also eliminates some of the gaseous and training substances. A number of equipment viz. LTLT (law temperature long time, 74°C for 30 min); HTST (high temperature short time, 85oC for 15s.) and Vacreator, a direct steam injection method, can be employed for this purpose. More severe heat treatment of cream should be avoided as higher, the temperature the greater the migration of copper from the milk serum into milk fat globules. This increases the level of copper associated with the milk fat making it more prone to the development of oxidative rancidity and reduce the shelf-life of butter.
Pasteurization of cream for making ripened cream butter is commonly carried out at higher temperature than for sweet cream butter e.g. 90-95oC for 15 or 105-110oC with no holding. Severe heat treatment denatures whey proteins, particularly lactoglobulins, exposing-SH groups which act as antioxidants and can enhance starter growth.
18.2.4 Ripening of cream
Ripening refers to the process of fermentation of cream with the help of suitable starter culture. This step can be eliminated if sweet-cream butter is desired. The main object of cream ripening is to produce butter with higher diacetyl content. Ripening improves the keeping quality of salted butter but it reduces the keeping quality of a salted butter. Starter culture consisting of a mixture of both acid producing (Streptococcus lactis, S.cremories) and flavour producing (S.diacetylactis, Leuconostoc citrovorum and/or Leuc. dextranicum) organisms is added. Amount of starter added depends on several factors and usually ranges between 0.5-2.0 percent of the weight of the cream. After being thoroughly mixed, the cream is incubated at about 21oC till desired an acidity is reached. Cream is subsequently cooled to 5-10°C to arrest further acid development.
Biosynthesis of diacetyl is not sufficient above pH 5.2. Stopping fermentation of cream by cooling at pH 5.1-5.3, results in a milder flavour; whereas continuing fermentation upto pH 4.5-4.7 results in higher levels of both diacetyl and lactic acid, giving more pronounced flavour.
18.2.4.1 Purpose
The fundamental objects of cream ripening are to produce butter with a pleasing, pronounced flavor and aroma, and to produce this flavor and aroma uniformly from day to day. Ripening also influences somewhat the exhaustiveness of churning and it affects the keeping quality of the butter variously, according to quality of original cream, churning acidity, and whether made into salted or unsalted butter.
18.2.4.2 Starter culture
Mixture of both acid producing organisms (Lactococcus lactis, L. cremoris) and flavour producing organisms (S. lactis subsp. diacetylactis, Leuconostoc citrovorum and/or Leuconostoc dextranicum). Starter culture is added at the rate of 0.5 to 2.0% of the weight of cream and incubated at about 21oC till desired acidity is reached. Usually it takes 15-16 hrs.
18.2.4.3 Effect
of Cream ripening on butter
Effect on Flavor
and Aroma
The mildly acid and pronounced “nutty” flavor that is characteristic of the pleasing flavor of good butter is usually accompanied by a high, attractive aroma. The typical butter flavor is due to the presence of diacetyl in combination with lactic acid, carbon dioxide, acetoin and intermediary products such as acetaldehyde, and probably other aromatic products as yet not definitely determined. These substances are the products of fermentation, brought about by the associative action of lactic acid-producing bacteria and citric acid-fermenting bacteria.
These bacteria are propagated and their flavor and aroma substances produced in the starter. During cream ripening the starter that is added to the cream, therefore, functions in two ways. It seeds the cream with species of bacteria that are capable of producing the desired aroma and flavor substances in the cream, and it adds to the cream the aroma and flavor substances produced and already contained in the starter.
Factors which
Influence the Diacetyl + Acetoin
Content of Butter
Cream ripened with a normal starter shows a varying ratio of diacetyl to acetoin. In the case of 20% cream, a ripening temperature of 17˚C. (62.6˚F) yields the largest amount of diacetyl. With increasing fat content the amount of diacetyl + acetoin increases. Cream testing 40% fat yields higher diacetyl content in the butter than 20% cream, or whole milk. The diacetyl content of butter to affect its flavor as follows:
|
Amount of Diacetyl |
Flavor |
|
Absence of diacetyl |
Flavorless |
|
0.2 to 0.6 ppm diacetyl |
Mild flavor |
|
0.7 to 1.5 ppm diacetyl |
Full flavor |
Distribution of Diacetyl + Acetoin by the
Churning Process
Butter contains a relatively small proportion of the diacetyl and acetoin content of the cream from which it is made. Fresh buttermilk contains larger amounts of diacetyl and acetoin than the corresponding butter and than the original cream, at churning time. The serum of butter contains larger amounts of diacetyl + acetoin than the fat of the same butter. The wash water contains appreciable amounts of diacetyl + acetoin. Churning 2 liters of cream yields the following amounts of diacetyl in the buttermilk, wash water and butter:
|
Butter milk |
6.14 mg Diacetyl |
|
First wash water |
3.16 mg Diacetyl |
|
Second wash water |
0.00 mg Diacetyl |
|
Third wash water |
0.00 mg Diacetyl |
|
Butter |
0.30 mg Diacetyl |
The following amounts of diacetyl + acetoin are present in washed and unwashed butter:
|
|
Diacetyl (mg/kg) |
Diacetyl + Acetoin (mg/kg) |
|
Unwashed Butter |
1.69 |
9.30 |
|
Washed Butter |
0.86 |
3.78 |
These findings indicate that in commercial manufacture of butter, particularly in the case of unsalted butter, excessive washing gives the finished butter, even when made from properly ripened cream, a disappointing “washed-out” flavor.
Effect of Cream Ripening on Keeping Quality of Butter
The development of flavor and aroma in butter by cream ripening, or by any other process of manufacture, can be of value only, provided that it does not impair or destroy the keeping quality of the resulting butter. The ripening of cream affects the keeping quality of butter in two fundamental ways, namely, by its control of age deterioration due to bacterial causes, and by its influence on age deterioration due to chemical causes.
Bacteriological
effect
The ripening of cream improves the keeping quality of butter as far as keeping quality is dependent on freedom from age deterioration due to biological causes. Cream ripening assists in controlling bacterial deterioration in butter. In butter made from ripened cream there is a great prevalence of lactic acid bacteria and a relatively high acidity and probably an abundance of lactate salts. These agencies are antagonistic to the great majority of flavor-damaging organisms that may be present in the butter, thus retarding their action, preserving the fresh or desired flavor, and prolonging the keeping quality of the butter.
Chemical
Effect
Cream ripening does not improve the chemical stability of butter. On the contrary, under average commercial conditions of manufacture, the ripening of cream to a full aroma and flavor shortens the life of salted butter. The usual flavor defects that develop with age in butter made from fully ripened cream are oily-metallic, fishy and sometimes tallowy flavor. There is a tendency also to intensify the well-known cold storage flavor. This is especially true of salted butter made from cream that arrives at the factory in sour fermented condition, is neutralized, pasteurized and re-ripened to a high acidity. It applies also, through to a somewhat lesser extent, to salted butter made from ripened sweet cream. It does not apply to unsalted butter.
Salted butter made from sweet, unripened cream, or from sour cream, neutralized and pasteurized, keeps better from the standpoint of absence of flavor deterioration due to chemical causes, than salted butter made from the same cream ripened to a full flavor and aroma.
18.2.4.4 Percent Acid to which the Cream should be Ripened
For fresh consumption salted butter, cream of moderate richness (30% fat) may safely be ripened to about 0.25 to 0.30% acid. For salted butter of commercial cold storage, it has been found preferable not to ripen the cream and to churn it at an acidity of about 0.21% acid or lower. In case of unsalted butter, the cream may be ripened to any acidity without jeopardizing keeping quality.
18.2.5 Cooling and ageing
Cooling and ageing are processes which prepare the cream for subsequent operation of churning. When cream leaves the pasteurizer, the fat in the globule is in liquid form. When cream is cooled, fat crystallization starts, cream will not churn unless the butter fat is at least partially crystallized. If solidification of fat is not sufficient, the fat losses in butter are high. Rate of cooling has an important influence on the body and texture of butter. The temperature to which cream is cooled is chosen is such a way that the butter produced is of optimum consistency and cream churns to butter in a responsible time of about 35-45 minutes. Churning at too high temperature may give butter with ‘greasy’ body which may work up too quickly and become sticky. Generally cooling temperature in summer should be 7-9°C and that if in winter (10°-13°C).
Crystallizing of the milk fat during aging
Before churning, cream is subjected to a program of cooling designed to control the crystallization of the fat so that the resultant butter has the right consistency.
Butter fat contains varying amounts of soft and hard fats. The relative amounts of fatty acids with high melting point determine whether the fat will be hard or soft. Soft fat has a high content of low-melting fatty acids and at room temperature this fat has a large continuous fat phase with a low solid phase, i.e. crystallized, high-melting fat. On the other hand, in a hard fat, the solid phase of high-melting fat is much larger than the continuous fat phase of low-melting fatty acids.
In butter making, if the cream is always subjected to the same treatment it will be the chemical composition of the milk fat that determines the butter's consistency. A soft milk fat will make a soft and greasy butter, whereas butter from hard milk fat will be hard and stiff.
Pasteurization causes the fat in the fat globules to liquefy. And when the cream is subsequently cooled a proportion of the fat will crystallize. If cooling is rapid, the crystals will be many and small; if gradual the yield will be fewer but larger crystals. The more violent the cooling process, the more will be the fat that will crystallize to form the solid phase, and the less the liquid fat that can be squeezed out of the fat globules during churning and working.
The crystals bind the liquid fat to their surface by adsorption. Since the total surface area is much greater if the crystals are many and small, more liquid fat will be adsorbed than if the crystals were larger and fewer. In the former case, churning and working will press only a small proportion of the liquid fat from the fat globules. The continuous fat phase will consequently be small and the butter firm. In the latter case, the opposite applies. A larger amount of liquid fat will be pressed out; the continuous phase will be large and the butter soft.
So by modifying the cooling program for the cream, it is possible to regulate the size of the crystals in the fat globules and in this way influence both the magnitude and the nature of the important continuous fat phase.
Treatment of hard fat
For optimum consistency where the iodine value is low, i.e. the butterfat is hard, as much as possible of the hardest fat must be converted to as few crystals as possible, so that little of the liquid fat is bound to the crystals. The liquid fat phase in the fat globules will thereby be maximized and much of it can be pressed out during churning and working, resulting in butter with a relatively large continuous phase of liquid fat and with the hard fat concentrated to the solid phase.
The program of treatment necessary to achieve this result comprises the following stages:
- rapid
cooling to about 8oC and storage for about 2 hours at this
temperature; heating gently to 20 - 21oC and storage at this
temperature for at least 2 hours (water at 27 - 29oC is used
for heating)
- cooling
to about 16oC
- Cooling
to about 8oC causes the formation of a large number of small
crystals that bind fat from the liquid continuous phase to their surface.
Treatment of medium-hard fat
With an increase in the iodine value, the heating temperature is accordingly reduced from 20-21oC. Consequently a larger number of fat crystals will form and more liquid fat will be adsorbed than is the case with the hard fat program. For iodine values up to 39, the heating temperature can be as low as 15oC.
Treatment of very soft fat
Where the iodine value is greater than 39-40 the "summer method" of treatment is used. After pasteurization the cream is cooled to 20oC. If the iodine value is around 39 - 40 the cream is cooled to about 8oC, and if 41 or greater to 6oC. It is generally held that aging temperatures below the 20o level will give a soft butter.
18.3Churning of Cream
It is during the
churning process that cream is converted into butter. Here the fat gloubles are disrupted under controlled conditions to
destabilize o/w emulsion and bring about agglomeration of milk fat. What
happens during churning has been explained by various theories of churning as
discussed in lesson 8.3. The sequence of events that occur during churning is
as follows:
i) Churning is initiated by agitation of cream causing incorporation of numerous air bubbles into the cream.
ii) With incorporation of air there is increase in the volume of cream and air plasma interface.
iii) Surface active (such as frictional, impact, concussion etc.) causes partial disruption of fat globule membrane
iv) The fat film, thus formed, serve as a foam depressant causing the air bubble to burst.
v) The liquid fat also serves as cementing material causing fat globules to clump together and eventually butter grains are formed which floats in plasma i.e. butter milk.
18.3.1 Initial working
Working of butter is essentially a kneading process in which butter granules are formed into a compact mass. During this operation, any excess moisture or buttermilk is removed. However, the emulsion (w/o) at this stage is not fully stable.
18.3.2 Salting of butter
In conventional process, butter may be salted by adding salt to butter churn after initial working of butter. Salt to be added must be high quality e.g. IS 1845:1961, with low level of lead, iron and copper. The grain should be fine, all passing through IS: sieve-85 (aperture 8424). It should be 99.5 to 99.8% sodium chloride and microbial count should be less than 10/g. Salt sets up osmotic gradient which draws water from the butter grains. This can lead butter to be leaky. Salted butter should therefore, must be thoroughly worked. Salt may be added either in dry form or as saturated brine solution.
18.3.3 Adjustment of moisture
After the
addition of salt, the moisture content in butter is adjusted by adding
calculated amount of additional water. In most countries, maximum
limits of 16% is placed on the level of moisture. Amount of water is to
be added in a batch of butter is calculated as follows:
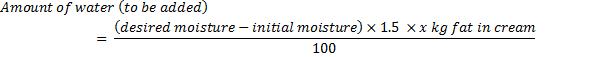
Starter distillates, if required, may also be added at this stage to enhance the flavour of resultant butter, if cream has not been cultured.
18.3.4 Final working of butter
The objective of working butter is to incorporate moisture and uniformly distribute added moisture and salt in butter. During this process remaining fat globules also break up and form a continuous phase, and moisture is finally distributed to retard bacterial growth in butter. It is safer to slightly over-work butter than to under-work. Under-worked butter may be leaky in body with large visible water droplets and may develop ‘mottles’ on standing. Moisture droplet size normally ranges from 1 to 15 micron and there are approximately 10 billion droplets per gram of butter. Working affects the colour of butter (making is slightly light). Working also increase air content (this favors growth of microorganisms, oxidative effects and therefore poor keeping quality). Vacuum working of butter may be carried out with advantage to reduce the air content of butter. Vacuum range from 15-40 cm of Hg may be used. Air content of conventional butter range from 3-7% by volume with an average of 4 ml/100 while that of vacuum worked butter it is about 1 ml/100g.