Module 3. Processing of fruits and vegetables
Lesson 11
DRYING: PRINCIPLE, METHODS AND APPLICATIONS
11.1 Introduction
Drying and dehydration of fruits and vegetables is an age old method to preserve these products. Removal of the water (75-90%) present in fresh commodity results in reduction in the water activity and ultimately resistance against most of the deteriorative agents. The removal of water is carried out by the application of heat and this heat is usually supplied in the form of solar energy or artificially generated hot air. Removal of moisture and exposure of heat often results in poor textural attributes, loss in nutritive value (vitamins), discolouration and loss of flavouring components. Although both drying and dehydration are interchangeably used, drying is referred to removal of water to an equilibrium moisture content while dehydration is removal of water to an almost bone dry condition.
A number of processing steps are carefully designed to check all these adverse effects of drying. Some of the new technologies have been introduced in recent years to produce a wholesome and nutritive product. Partial dewatering by osmosis and impregnation soaking process before drying saves energy during drying and improves quality of dried product. Osmotic dehydration is gaining popularity, as the dehydrated product is more stable during storage due to low water activity by solute gain and water loss. The low water activity resulted in fewer rates of chemical reactions avoiding deterioration of the food. Osmotic dehydration in many cases is employed to increase sugar to acid ratio of acidic fruits, thereby to improve the taste, texture and appearance of dried product. The processing steps involve in drying of fruits and vegetables are summarized here.
11.2 Why Drying of Foods?
· Water activity is defined as the ratio of vapour pressure of food to that of the vapour pressure of pure water at a constant temperature. Reduction in water activity (aw) so control/check over chemical and microbiological changes (deterioration).
· Reduction in weight, size and volume of the food material. Hence bulk transportation becomes easier and cheaper.
· Packaging requirements are simple and cheap.
· Facilitate further processing. Example: grain drying for flour.
11.2.1 Water content in foods
Water is present in food as free or/and bound water. Free water is defined as water within a food that behaves as pure water. Unbound water is removed during the constant rate period of drying, when the nature of food does not have a great effect on the drying process.
Bound water can be defined as water that exhibits a lower vapour pressure, lower mobility and greatly reduced freezing point. So, bound water molecules have different kinetic and thermodynamic properties than ordinary water molecules. The aw as affected by the extent of bound water is given in Table 11.1:
Table 11.1 Water activity as affected by the extent of bound water
|
Extent of bound water |
Water activity (aw) |
|
Tightly bound water |
< 0.3 |
|
Moderately bound water |
0.3 to 0.7 |
|
Loosely bound water |
> 0.7 |
|
Free water |
~ 1.0 |
11.3 Mechanism of Drying
When hot air is blow over a wet food, heat is transferred to the surface and latent heat of vaporization causes water to evaporate. Water vapours diffuse through a boundary film of air and is carried away by the moving air. This create a region of lower water vapour pressure gradient is established from the moist interior of the food to the dry air. The gradient provides the “driving force” for water removal from the food.
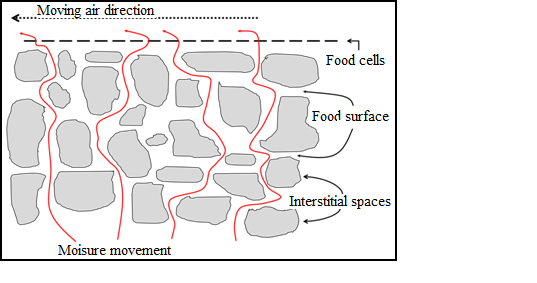
Fig 11.1 Schematic diagram of movement of moisture in the interstitial spaces of food cells during drying in fruits and vegetables
1.
Liquid movement
by capillary force.
2.
Diffusion of
liquids, caused by concentration gradient.
3.
Diffusion of
liquids, which are absorbed in layers at the surfaces of solid components of
the food.
4.
Water vapour diffusion in air spaces within
the food caused by vapour pressure gradients.
11.3.1 Phases of drying
1. Initial
warm up period
2. Constant
drying rate period
3. Falling
drying rate period
In hygroscopic food material more than one falling rate period occurs. In the first period plane of evaporation moves inside the food and water diffuses through the dry solids to the drying air. It ends when plane of evaporation reaches to the centre of food and the partial pressure of water falls below the saturated water vapour pressure. Second falling rate period occurs when the partial pressure of water is below the saturated vapour pressure and the drying is by desorption. Falling rate period is the longest period during drying of food product. Equilibrium moisture content (EMC) occurs when dry spots develop at the surface so less area exposed to dry air and evaporation decreases.
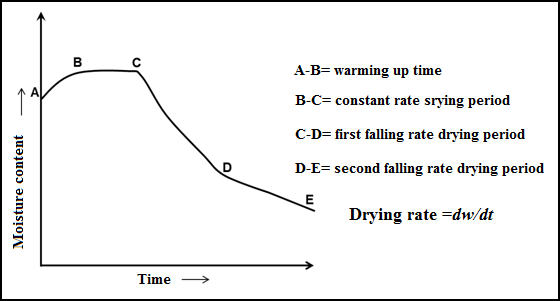
Fig 11.2 Schematic diagram of change in moisture content with time (drying rate)
There are 4 resistances to heat transfer in drying:
1.
Resistance to
external heat transfer
2.
Resistance to
internal heat transfer
3.
Resistance to
external mass transfer
4.
Resistance to
internal mass transfer
11.4 Drying Time
Drying time is
the total time required for removal of water from food material. Drying time during
constant rate period is inversely proportional to wet bulb depression
while drying rate is directly proportional to wet bulb depression.
![]()
where, wi = initial moisture content, wt = moisture content at time t, ρs = bulk density of solid, L = latent heat of vapour, d = thickness of material, h = surface heat transfer coefficient, Ta = dry bulb temperature of hot air, Tw = wet bulb temperature of air, (Ta-Tw) = Wet bulb depression
If velocity of air is increased, h and hence drying rate will increase and drying time is decreased.
Total drying time is the time required to attain critical moisture content (wt = wC)
Drying time during
falling rate period is decided by predominantly by diffusion and to
some extent by capillary action.

where, D = liquid diffusivity
we = equilibrium moisture content
![]()
if, wi < wc it is falling rate
wi > wc it is constant rate
11.5 Factors Affecting Drying Rate
The factors that affect drying rate are external and internal factors.
The external factors are:
· Dry bulb temperature
· Relative humidity
· Air velocity
· Surface heat transfer coefficient
The Internal factors are:
· Surface to volume ratio
· Surface temperature
· Rate of moisture loss
· Composition i.e. moisture, fat
11.6 Effects of Drying on Foods
11.6.1 Shrinkage
During drying as moisture is removed and food material becomes smaller in size. This also affects bulk density (weight per unit volume) of food material. Slow drying results in development of internal stress. These rupture compress and permanently distort the relatively rigid cells, to give the food a shrink / shrivelled appearance. Such food material on rehydration absorbs water more slowly. Gelatinization of starch, denaturation of proteins, and crystallization of cellulose also affect rehydration characteristics. Rapid drying improves textural characteristics such as wettability, sinkability, dispersibility and solubility.
11.6.2 Case hardening
Formation of impervious layer over the surface of a dried food product characterized by inner soft and outer hard layer resulting in inadequate drying. It always occurs in food products rich in solutes and when initial drying temperature is very high. During the initial high temperature solute particles comes out and deposit at the surface resulting of building up of an impermeable layer which prevents further moisture removal. It can be prevented by using lower drying temperature.
11.6.3 Browning
Browning refers to change in the colour of food material to light to dark brown colour. This change in colour may occur by any of the three methods given below.
· Residual enzymatic browning: the residual enzymes especially in vegetables such as polyphenol oxidases cause oxidation that result in the change of colour.
· Maillard’s reaction: it is the reaction between the amino group of proteins and reducing sugars of carbohydrates in presence of heat. This type of browning is most common in dried foods.
· Caramelization: it is the conversion of sugars only into dark coloured compounds in presence of heat.
11.7 Types of Drying
a. Hot Air drying: It includes spray drying, tray drying, fluidized bed drying, etc. In spray drying a fine dispersion of pre-concentrated food (40-60% moisture) is “atomized” to form fine droplets and then sprayed into a co-or counter-current flow of heated air at 150-300°C in a large drying chamber. Tray driers consist of an insulated cabinet fitted with shallow mesh or perforated trays, each of which contains a thin (2-6 cm deep) layer of food. Hot air is blown at 0.5-5.0 m/s through a system of ducts and baffles to promote uniform distribution over and/or through each tray.
b. Microwave drying: It involves use of microwaves.
c. Freeze drying: It is also known as “lyophilization” and is usually used for drying heat sensitive food material by freezing the material and then reducing the surrounding pressure to allow the frozen water to sublimate directly from the solid phase (ice) to gas phase (water vapour).
d. Osmotic drying: This is explained in section 11.8
11.8 Osmotic Dehydration
Osmotic dehydration, also called as dewatering and impregnation soaking (DIS) process, was pioneered by James D. Ponting in 1966. It is a water removal process that involves the soaking of foods – mostly fruits and vegetables in hypertonic salt or sugar or in a combined solution, to reduce the water content while increasing the soluble solid content. Osmotic dehydration is undertaken to reduce the product water activity in minimal processing, which is carried out either at atmospheric pressure or at vacuum conditions. The raw material is placed in concentrated solutions of soluble solids with higher osmotic pressure and lower water activity. Water moves through the selective permeable membrane with much greater ease than in the dissolved substances.
11.8.1 Mechanism of osmotic dehydration
In osmotic dehydration process, a solid product of high moisture content is immersed in a concentrated solution (mainly of sugar or salt), which initiates three types of counter-current mass transfer. The diagrammatic presentation of osmotic dehydration is given in Fig. 11.3.
· Water outflow from the product to the surroundings solution as a result of osmosis through a semi-permeable membrane.
· Solute transfer, from the solution to the product.
· Leaching out of the water-soluble component along with water from the product to the solution.
The last two mass transfers occur mainly because of diffusion. This counter current mass flow is due to the water and solute activity gradients across the cell’s membrane. This process continues till the osmotic potential on two processes reaches equilibrium. In an ideal osmotic solution a semi-permeable membrane would be permeated by the solvent molecules but not by the solute molecules. In fruits and vegetables, the cell wall membranes are living biological units and selective permeable, which can stretch and expand under the influence of growth and turgor pressure generated inside the cells. These cellular membranes, which are composed mainly of parenchymatous cells, freely allow the solvent molecules to pass through, but they also allow, to a lesser degree, the passage of some of the solute molecules. Such membranes are called as differentially permeable, rather than semi-permeable. Osmotic dehydration may result in 40-50% decrease in initial volume, if properly performed.
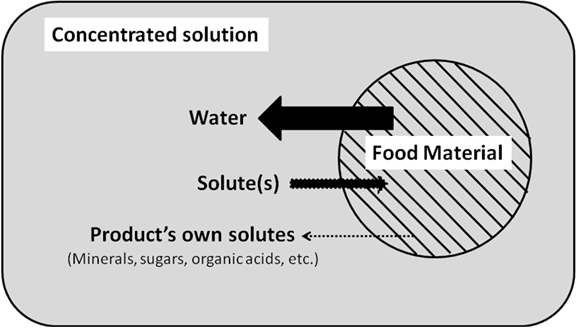
Fig.11.3 Diagrammatic presentation of Osmotic Dehydration
11.8.2 Osmotic agents and their requirements
Osmotic agent must have lower water activity (aw), good solubility, constant concentration during processing, and it should be cheap. However, non-toxicity, inertness to food constituent and good sensory attributes is other added attraction, while selecting any osmotic agent. These are number of compounds available, satisfying above mentioned criteria, like, sucrose, glucose syrups, invert sugar, corn syrups, honey, and humectants such as sorbitol and glycerol. The kind of sugar utilized strongly affects the kinetics of water removal, and by increasing the molecular weight of osmotic substance, larger water removal could be achieved with little uptake of solutes. Low molecular weight substances (glucose, fructose, sorbitol etc.) favour the sugar uptake because of the high velocity of penetration of the molecules so that solid enrichment instead of dehydration is the main effect of the process. Sodium chloride (NaCl) is an excellent osmotic agent for vegetables and other animal derived products, but its use with fruits is restricted because of alteration in taste and bleaching of colour. Addition of NaCl to osmotic solution increased the driving force for drying owing to the aw lowering capacity of salt. Synergistic effects between sugar and salt have also been observed. The use of blends comprising two or more solutes seems to be an attractive alternative.
11.8.3 Factors affecting osmotic dehydration
In any type of food product, many mechanisms can be acting at the same time, the relative contribution of which depends upon the following:
1. Nature of the product
2. Processing temperature
3. Operating conditions such as osmosis, diffusion, flux interactions and shrinkage.
11.9 Manufacturing Steps Involved In Drying of Fruits And Vegetables
11.9.1 Selection of raw material
The major cost involve in drying operation is the price of raw material. Hence good quality raw material is of prime importance. Immature or over mature fruits and vegetables often result in poor quality product. For example over mature green peas result in less sweet and starchy dried product with poor rehydration characteristics. Similarly high specific gravity fully mature potatoes with low reducing sugar content are desirable because they are large, mealy and less prone to browning or yellowing during dehydration.
11.9.2 Washing
Before further processing raw materials should be thoroughly washed to remove the adhering dirt, dust and other foreign particles. On commercial scale raw material is dumped in large tanks for some time and then sprayed with a jet of water. Alternatively, it may cleaned in a stream of running water. Water for this purpose must be of potable quality. To remove the traces of pesticides, colouring material commonly used nowadays to impart shining; some of the chemicals like dilute acids may also be added in washing water. Addition of sanitizers like chlorine based compounds is permitted to certain level for washing purpose. These sanitizers improve the microbiological quality of the finished product.
11.9.3 Peeling, trimming and sizing
Fruits and vegetables are either dried as whole or undergo size reduction before drying. A number of peeling processes are available on commercial level. Trimming is done to remove unwanted parts. Sizing is done to develop uniform product and it also facilitates subsequent unit operation. Increase in surface area causes faster drying.
11.9.4 Pre-treatments
In order to improve the quality of finished product fruits and vegetables they are invariable subjected to many pre-treatments. Some of the pre-treatments are summarized here.
11.9.4.1 Checking
Checking is an operation in which fruits specially raisins, prunes are dipped for a brief period in hot solution of alkali. This cause removal of waxy layer and it also improve the drying rate.
11.9.4.2 Blanching
Blanching is mild heat treatment given to some fruits and majority of vegetables primarily to inactivate the naturally occurring enzymes. These enzymes belong to peroxidase group like polyphenol peroxidases that catalyze the oxidation of phenolic substances, resulting in production of brown coloured compounds. The cell wall degrading enzymes, catalase (off-flavour), and ascorbic acid oxidase also get inactivated during blanching process. Blanching improves the colour of dried products, it aids to rapid reconstitution of dried product, it also increase drying rate, it also expels dissolved oxygen and it also improve bacteriological quality of the finished product.
11.9.4.3 Sulphiting
Sulphur dioxide and sulphites (usually sodium metasulphite or potassium metabisulphite) are well known anti-browning agents. The sulphur dioxide gas competes with peroxidases for the active sites and prevents the oxidation of phenolic substances. In many products that contain anthocyanin as major pigment, the colour of the product turned blue when exposed to acidic conditions. In such fruits or vegetables sulphur dioxide treatment may prove beneficial. The blanched or checked fruits or vegetables are exposed to fumes of sulphur dioxide by burning sulphur powder in an enclosed chamber. This is most widely used method of sulphuring. However, they can be dipped in solution of some sulphur salt. Proper penetration of sulphur dioxide is important to prevent internal darkening during drying. In fuming process, the sulphur dioxide absorption is faster as compared dipping. However the absorption is more uniform in later.
11.9.5 Drying
Drying of the fruits and vegetables is usually carried out in cabinet dryer. The material is loaded over perforated aluminium trays and dried using hot air. The temperature, velocity and the humidity of the air are important for drying process. It usually varies from commodity to commodity. A lower drying temperature is generally used for fruits, as there may be chances of case hardening. The temperature varies from 55-80oC. To create perforation initially temperature is maintained towards higher side and them it is reduced. Some vegetables those are light, cylindrical or spherical like peas are dried in fluidized bed dryer. This improves the rehydration characteristic and the nutritive value of the product, as it take lesser time and more uniform drying.
11.10 Some Common Processes Used for Drying of Fruits and Vegetables
11.10.1 Drying of some vegetables
11.10.1.1 Drying of onion
Onion is bulbous crop characterized by pungent flavour, attractive colour and high amount of reducing sugar. It an important seasoning item for traditional Indian cuisines. The demand for onion has increased considerably over the years because of its unique flavour profile and well established medicinal and therapeutic properties. To meet the demand of consumers, the onions are processed in various convenient forms as paste, dried slices powder, etc. The flow diagram represents the various unit operations involved in drying of onion.

11.10.1.2 Drying of cauliflower
Cauliflower contains protective nutrients and small quantities of major nutrients. The storage life of cauliflower is 2 to 4 weeks at 0oC. However, the preservation of cauliflower is possible by canning, freezing, drying and pickling.
During drying or canning, white cauliflower is turned to pink or brown discolouration, which seriously affects the acceptability. The pigmentation of cauliflower is attributed mainly due to the presence of leucoanthocyanidins. The discolouration can be checked by addition of 0.05% citric acid and 0.03% ascorbic acid. Blanching treatment of cauliflower containing 0.075% sodium metabisulphite and 0.25% sodium sulfite followed by dipping in 20% glycerol or sugar syrup resulted in a superior dried product of pale white colour with superior rehydration characteristics.
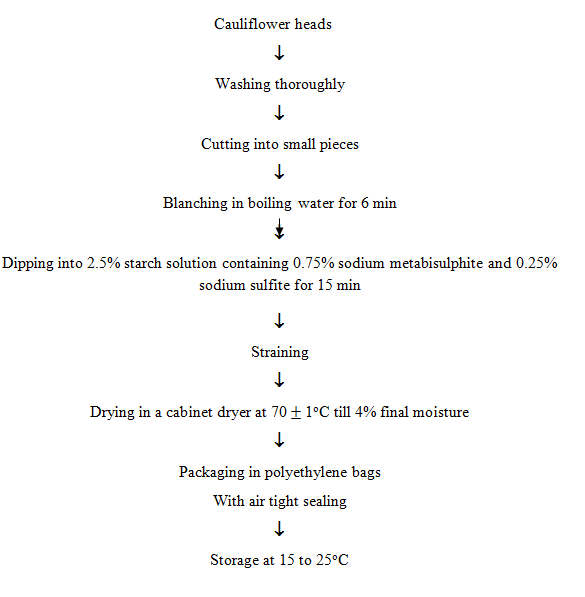
11.10.2 Drying of fruits
Perceived as a “value-added” ingredient, dried fruit adds flavour, colour, texture and diversity with little alteration to an existing formula. The growing interest in dried fruits and the change to a more healthy way of eating has also moved dried fruits considerably closer to the mainstream.
Found primarily in the baking industry, dried fruits is coming into its own in various food products, including entrées, side dishes and condiments. Compotes, chutneys, rice and grain dishes, stuffings, sauces, breads, muffins, cookies, deserts, cereals and snacks are all food categories encompassing dried fruit.
Since some dried fruits is sugar infused (osmotic drying), food processors can decrease the amount of sugar in formula – this is especially the case in baked products. Processors are making adjustments in moisture content of the dried fruits so that a varied range is available for different applications. An added bonus is dried fruits’ shelf stability (a shelf life of at least 12 months). Dried fruit is more widely available in different forms, including whole dried, cut, diced and powders.
11.10.2.1 Drying of apple
