Module 6. Meat and
poultry processing
Lesson 22
RIGOR MORTIS: BIOCHEMICAL AND
HISTOLOGICAL CHANGES
22.1 Introduction
Meat is basically defined as the flesh of animals used as food. The term meat generally differs from the muscle in the sense its structural and physicochemical nature as it (muscle) has undergone certain chemical and biochemical changes following death of an animal which is a postmortem aspect. Thus, during the time elapsed between death of an animal and its processing, a series of biochemical and physico-chemical changes takes place which lead to conversion of muscle into meat.
22.2 Muscle: Structure, Composition & Functioning
Muscle is made of number of fiber bundles (1.0 mm thick), comprised of a group of fibers, (0.1 mm thick) held together by a structure of connective tissues or perimysium (figure 22.1). Connective tissues which provide edible texture, structure and flexibility to the muscles, comprised of fibrous protein collagen, reticulin, and elastin. Muscle fiber, a unit of muscle contraction, is a multinucleate, cylindrical cell bounded by an outer membrane or sarcolemma and is consist of myfribils of 1-2 micron size. Myofibrils are separated by sarcoplasmic reticulum, a fine network of tubules. Each fiber is filled with sarcoplasm containing mitochondria, enzymes, glycogen, ATP, creatine, and myoglobin. The myofribrils are cross striated to give rise to understanding of physical structure of muscles (dark or A and light or I/Z bands). The unit of fibril is sacromere which lies between adjacent two Z- bands. Fibrils are consist of two set of filaments i.e. myosin and F-actin. Contraction and relaxation of striated muscles takes place due to interaction between actin, myosin and ATP. In the presence of magnesium and calcium ions, myosin liberates ATP which results in muscle contraction.
The composition of muscle is highly variable depending upon specie, type of muscle, animal’s maturity and the treatments given to the animal before its slaughtering. Variation in the composition ultimately affects the nutritional and functional profile muscle tissues.
The lipid components of muscle tissue vary more widely than do the amino acid in fish muscle, the differences have been made in the concept of lean or white fish and fatty fish. In lean fish, storage fat is carried in the liver. Muscle of lean fish contains <1% lipid, mostly phospholipids, located in the membrane. In fatty fish, depot fat apparently occurs as extracellular droplets in the muscle tissue.
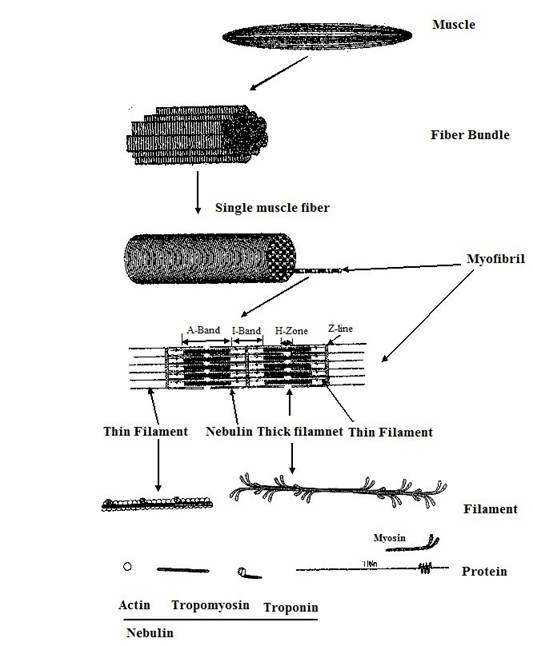
Fig. 22.1 Structure of Muscle (Redrawn and modified from the Greaser et al. 1999)
In mammals and birds, both the amount and type of collagen have important influence on textural properties of the muscle. In fish however, collagen is readily softened by normal cooking procedures.
Nature of muscle-
I) Striated or voluntary muscle –lean meat.
II) Unstriated or involuntary muscle- stomach wall.
III) Cardiac muscle- heart wall.
In white muscle, fat is apparently diffusely located among the muscle cells. Basically the lipid composition of meat of mammalian and avian muscles can be categorized into lipids from muscle tissue and lipids from adipose tissues lipid in the lean portion contains greater portions of phospholipids than lipids than lipids in adipose tissue lean muscle contains about 0.5-1.0% phospholipids and the fatty acids of phospholipids are more unsaturated than those of triglycerols. Consequently, lipid in the lean portion of meat has a higher degree of unsaturation than those in adipose tissue. The degree of unsaturation of fatty acids in cold blooded fish is much greater than that of fatty acids in avian and mammalian muscles. The much greater percentage of polyenoic fatty acids is found in avian and mammalian muscles. The much greater percentage of polyenoic fatty fish reflects differences in phospholipid – triacylglycerol ratios. Poultry fat is more unsaturated than pork fat, beef and mutton.
Table 22.1. Composition (%) of muscle tissue
|
SPECIES |
WATER (%) |
PROTEIN (%) |
LIPID (%) |
ASH (%) |
|
Beef |
70-73 |
20-22 |
4-8 |
1.0 |
|
Pork |
68-70 |
19-20 |
9-11 |
1.4 |
|
Chicken |
73.7 |
20-23 |
1.0 |
- |
|
Lamb |
73.0 |
20.0 |
5-6 |
1.6 |
|
Cod |
81.2 |
17.6 |
0.3 |
1.2 |
|
Salmon |
64.0 |
20-22 |
13-15 |
1.3 |
22.3 General Consequences Following Death of an Animal (Post Mortem Changes)
Following the death of animal, circulation of the blood ceases resulting in the complex series of changes within the muscle (Fig.22.2). As much as possible blood is removed from the animal carcass to increase the edibility and keeping qualities of the meat, since blood is an ideal medium for the growth of spoilage microorganisms. Failure of blood circulation and its removal from the muscle tissue results in depletion of oxygen supply to the tissue leading to depletion of ATP and creatine phosphate levels (due to stoppage of electron transport chain and oxidative phosphorylation) and most importantly to onset of anaerobic metabolisms of glycogen. Anaerobic metabolism of glucose and breakdown of ATPs by the continuing action of sarcoplasmic ATPase leads to depletion of ATP and creatine phosphate results in onset of rigor mortis on the other hand breakdown of glycogen in the absence of oxygen leads to production of lactic acid thus decrease in pH. Other postmortem physical changes in muscle are:
22.3.1 Change in pH
Decrease in pH due to lactic acid formation is accompanied by various exothermic reactions such as anaerobic glycolysis. pH changes from physiological pH i.e 7.2-7.4 to ultimately post-mortem pH i.e 5.3-5.5 in 24 hrs. This has profound effect on muscle portion of meat. Usually glycolysis ceases even before the glycogen is depleted.
22.3.2 Change in temperature
Temperature of animal increases from 37.6-39.0°C. This is the reason why animal cools slowly during refrigeration as a result of continuous production of heat. This phenomenon is known as “animal heat”. Removal of “animal heat” by chilling or refrigeration is essential to ensure longer shelf-life of meat.
22.3.3 Change in proteins
Due to the change in pH and high temperature, colour of meat changes and water holding capacity (WHC) also decreases. Sarcoplasmic proteins get denatured and attached to the surface of myofilament, which produces change in meat colour which becomes light. Water holding capacity of myofibril proteins decreases resulting in exudation of fluid.
Sarcoplasmic proteins are more lable with respect to physiological conditions prevailing in the post- mortem muscles. These proteins are highly susceptible to disruption as compared with myofribrilar proteins. Sarcoplasmic proteins during rigor mortis denatured below pH 6.0 and at 37°c.
22.3.4 Change in water holding capacity
Water holding capacity is the function of respective proteins which binds with water. In pre-rigor stage meat possesses a high water holding capacity but later it decreases during first hour following death of animals. Lowest water holding capacity is found at its iso-electric pH i.e. 5.3-5.5. After post-rigor aging water holding capacity. is found to be increased because of increase in osmotic.
During post mortem movement of Na. K, Mg, and Ca in muscles takes place. But during aging there is continuous release of of Na & Ca. and uptake of K ions continued up to 6-8 days. The movement of cations produces an increased electrical charge on muscle protein which facilitated the formation of hydrated ions. This is believed to be the reason of increased water holding capacity during aging of meat.

Fig. 22.2 Generation consequences following death of an animal (adapted from Lawrie, 1966 and Eskin et al., 1971)
22.3.5 Post mortem glycolysis
After the death of animal, blood circulation stops, thus oxygen supply to muscles tissues decreases hence anaerobic conditions prevails in the muscle. The glycogen present in tissues is no longer converted into CO2 and water instead, converted in to lactic acid through anaerobic glycolysis. The conversion of glycogen takes place through two different pathways.
(a) Amyloytic pathway i.e. hydrolytic
(b) Phosphorolytic pathway
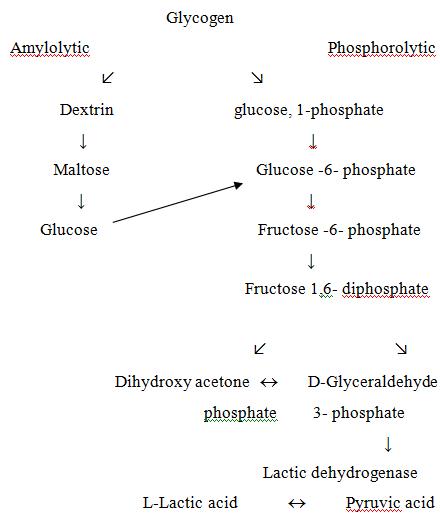
Fig. 22.3 Post mortem glycolysis
Due to this glycolysis pH changes from physiological pH i.e. 7.2-7.4 to ultimately post- mortem pH i.e. 5.3-5.5. This pH is attained within 24 hours and being related to ATP production, which falls in this pathway. The net fall in ATP is responsible for onset of rigor mortis. The pH 5.3-5.5 is ideal pH which can be obtained by well rested and well fed animal before slaughter (fig. 22.3).
22.4 Conversion of Muscle into Meat (Rigor Mortis)
The most important change that occurs in postmortem muscle is the development of rigor mortis, means stiffness of the muscle. The primary cause of onset of rigor is post mortem decline in the level of ATP. The process takes from 7-24 hrs depending on the species; however it is linked with the rate of depletion of ATP in muscle. The entire process of conversion of muscle into meat is broadly divided into three stages:
22.4.1 Pre-rigor stage
During early stages of postmortem or pre-rigor stage, the concentration of ATP more or less remains constant as the muscle tries to maintain ATP levels by an active creatine kinase. However it will lead to liberation of creatine from muscle. Thus, in this state creatine phosphate levels fall more rapidly than that of ATPs. ATPs are providing cushioning effect for the filaments of two proteins i.e. actin and myosin. This results in a meat which is soft and pliable. In pre-rigor stage, myosin dissociates from actin and can be extracted in solution of high ionic strength. Water holding capacity of the muscle proteins remains high during this stage.
22.4.2 Rigor mortis
This period is very important as meat becomes rigid and stiff. Onset of rigor mortis may be 8-10 hours postmortem and it may last in 15-20 hours in meat. Onset of rigor is demonstrated by fall in ATP, loss of extensibility of muscles and contraction of tissues. Time difference between death of an animal and onset of rigor state is termed as ‘delay phase’. This period depend upon number of factors such as age, health, size of carcass, the amount of fat cover, nutritional status of animal, pH and glycogen level, temperature as well. ATP plays very important role in this stage. As ATP level falls two muscle proteins gradually forms an associated actomyosin complex which is inextensible and is responsible for contraction. This is the necessary criterion for development of rigor mortis. The extent of contraction of the muscles is determined by estimation of length of the sacromere within the myofibril. Meat which is cooked in this state is very tough in texture. The water holding capacity of the muscle protein remains minimum during this stage due to drop in pH as it comes closer to their iso-electric point i.e. pH 5.3-5.5. If the ultimate pH (5.60) falls too quickly, carcass would still be warm adversely affecting water holding capacity and prevailing partial denaturation of protein resulting into pale, soft and exudative (PSE) muscle ultimately leading to lower yield of the meat. This is often encountered in pigs having sufficient reserves of glycogen. On the other hand if inadequately feed or fasting animal having minimum reserve of glycogen is subjected for slaughtering; dark, firm and dry (DFD) meat conditions. DFD meat is having pH not below 6.0 and is darker in colour and susceptible for microbial growth.
22.4.3 Post rigor (conditioning/ageing)
During post rigor stage meat become tenderizes and organoleptically acceptable when it is kept cold for sometime after rigor mortis. The muscle again becomes soft and pliable with improved flavour and juiciness. The post rigor meat provides lesser problems in toughness, when cooked compared to with that cooked in rigor. Meat gradually reaches to an optimum tenderness period after an ageing period of 10-18 days stored at 0°- 5°C following the dissolution of rigor. However, prolonged storage of meat in some species may results in some problems viz. microbial spoilage, desiccation of proteins, and development of off flavours. Thus it is recommended to consume meat before it gets spoiled. The ageing which also called as conditioning or ripening of meat is sometime accelerated by raising storage temperature for e.g. holding meat at 15° for 3 days period in UV to control the microbial growth at surface. While in the case of pork, ageing is not recommended rather to eat fresh as it develops rapid onset of fat rancidity even at low temperature. On the other hand beef is generally aged and lamb & mutton are occasionally aged.
Ageing is considered as
very important aspect of meat processing as it imparts desirable flavour,
textural and other sensory attributes to the finished product. The responsible
factors for this desirable changes are still been researchable issue, however
it is now a fact that in post- rigor state actomyosin complex does not
dissociate but other subtle changes occur like, increase in the water holding
capacity due to increase osmotic pressure in the muscle fibre due to net inside
movement of cations and breakdown of proteins by liberated proteolytic enzymes,
the cathepsins may lead to tenderness. While cooking of meat
tenderizing agents such as enzyme calpain etc are added which breaks down the
stiff muscle protein to yield a soft and orgnoleptically acceptable meat.