Module 1. Basic concept of thermodynamics
Lesson 1
THERMODYNAMIC SYSTEM & ITS PROPERTIES
1.1 Introduction
The field of science, which deals with energies possessed and exchanged by a system of gases and vapors, their conversion in terms of heat and work and their relationship with properties of system is called thermodynamics. Thus in thermodynamics, we deal with conversion of energy from one form to the other, mainly heat into work or vice versa. In this context, we must know the overall scenario of conversion of available forms of energy into usable forms as shown below:
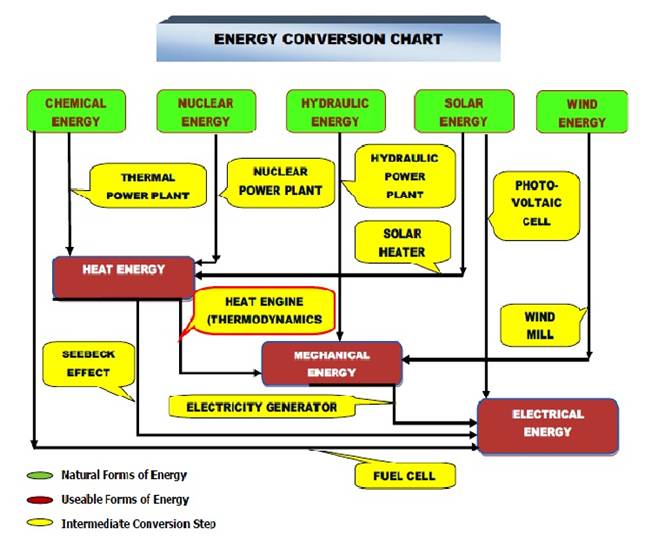
Fig.1.1 Energy conversion chart
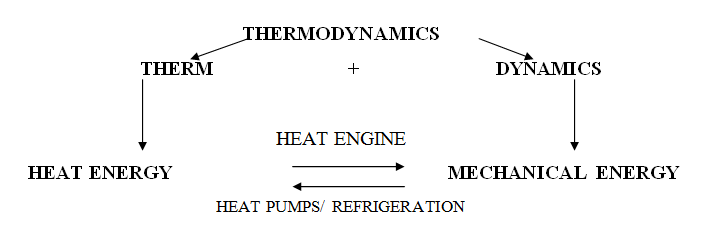
1.2 Application of Thermodynamics
Ø Power Generation (Thermal/Nuclear Power plants)
Ø Automobiles (Petrol/ Diesel/LPG/CNG engines)
Ø Processing Industries (Steam generation, Refrigeration systems)
Ø Gas Compressors (Air compressors)
As far as a dairy/food processing industry is concerned, we know that heating and cooling are two main processes. Heating is generally carried out by steam which is separately prepared in an industrial boiler by combustion of coal/oil. Thus chemical energy of fossil fuels is converted to heat energy which is carried to the required place by steam. Cooling is provided by means of mechanical refrigeration equipment which consumes electricity and produce cooling. During the power off period, electricity is generated by a generator, which is run by a diesel engine. The transport tankers are also driven by a petrol/diesel engine. These engines burn petrol/diesel/gas and convert heat energy produced into mechanical power to be used in any application. In a plant, compressed air is also required to operate different types of automatic valves and other instrumentation. Air is compressed by a mechanical compressor, which converts mechanical energy into pressure energy. In this way, the application of thermodynamics comes in the study of all said equipment like boilers, refrigeration plants, air/refrigerant compressors, and internal combustion engines etc which are extensively used in a dairy processing plant.
1.3 Thermodynamic System
It refers to a space or any matter (gas/vapor) within a prescribed boundary (which may be real or imaginary) upon which attention is focused for study e.g. a gas bounded by inner surface of a vessel may be called a thermodynamic system. This system carries out the job of energy conversion. A thermodynamic system may be of various types:-
a) Closed system
b) Open system
c) Isolated system
d) Adiabatic system
e) Homogeneous system
f) Heterogeneous system
(Fig. 1.2 :Thermodynamic system)
a.Closed system is one, the boundary of which, are closed or continuous so that no mass can enter or exit out of the system. However, the energy transfer between system and its surroundings can take place across the boundary of system.
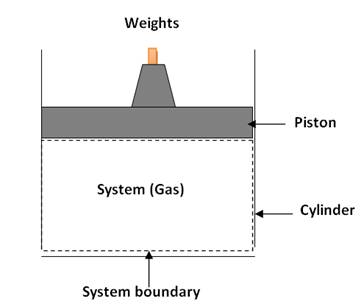
Fig. 1.3 (a) Closed thermodynamic system
For example: A thermos-flask filled with
hot tea - over a period of time, the content gets cooled because the heat is
transferred to the surroundings.
b. Open
system is one, the boundary of which, is not closed or
continuous, but has one or more openings through which mass transfer may also
take place in addition to energy transfer between system and its surroundings
e.g. a compressor shown in Fig. 1.3 (b)
is an open system. A water pump is also an example of open system.
If rate of mass & energy transfer
is constant it is called steady flow system, otherwise it is called unsteady
flow system.
c. Isolated
system: A system, which
is not influenced by surroundings in any way, i.e. neither the mass nor the
energy crosses the boundary of the system and it remains isolated from its
surroundings e.g. a system with completely enclosed and insulated boundaries.
d. Adiabatic system:
A system which is thermally insulated at its boundaries such that it is unable
to exchange heat energy with its surroundings is called adiabatic system.
However, an adiabatic system can exchange work or mechanical energy.
e. Homogeneous system:
A system in which the working medium is chemically and physically homogeneous
throughout is called homogeneous system.
f. Heterogeneous system: A system which consists of two or more, physically or chemically different phases of working medium is called heterogeneous system e.g. water and steam or water and oil.
1.3.1 Thermodynamic medium or working medium
Every thermodynamic equipment or system makes use of a working substance which acts as a medium for energy conversion. For example in IC engine, the medium used or working substance is air and fuel vapors, in power generation it is steam, in refrigeration system it is any refrigerant like R-11, R-12, ammonia etc. This medium is named as working medium and chosen for any specific thermodynamic application because of its specific properties. Thus the choice of working medium in a thermodynamic system or equipment is very much important.
1.3.2 Equilibrium of a system
A system is said to be at equilibrium if it does not tend to undergo any further change of its own accord. This equilibrium is between the system and its surroundings. Type of equilibrium depends on type of change as given below:
· Mechanical equilibrium is reached when there is no unbalancing of forces between system and surroundings.
· Thermal equilibrium is reached when there is no change in temperature of system with respect to time.
· Chemical equilibrium is reached when there is no chemical change in system with respect to time.
1.4 Properties of a System
A property of a system is any characteristic of the system which can be measured directly or indirectly, while the system remains at equilibrium. The properties of a system may be classified into two groups as:
(a) Intensive or Intrinsic properties
(b) Extensive or Extrinsic properties
(a) Intrinsic properties are those properties which are independent of the mass of system. These properties are inherent and do not depend on mass of the system e.g. temperature, pressure, specific volume, density, viscosity etc.
(b) Extrinsic properties are those which are dependent on mass of the system. Values of these properties depend directly on mass of the system. Value of extrinsic properties increases with increase in mass of system e.g. total or net volume, energy, weight.
1.4.1 Temperature (t)
It determines the level of heat intensity or thermal energy of a body/system. The difference in temperature of two bodies shows that the body with higher temperature is hotter than the body with lower temperature or the body with lower temperature is colder than the body with higher temperature. It is measured with the help of a thermometer which may be of many types.
The commonly used unit of temperature is “Celsius (°C)”. 10C is 1/100th of the difference between boiling point & freezing point of water where freezing point is taken as 0ºC. Another unit of temperature used in English speaking countries is “Fahrenheit”.
Temperature in °F = 1.8 x Temperature in °C + 32
In SI system of units the unit of temperature used is Kelvin (K), which is actually the unit of absolute temperature as mentioned below:
1.4.1.1 Absolute temperature (T)
The zero chosen on Celsius or Fahrenheit
scale is quite arbitrary and these scales are useful only when we have to
consider the change in temperature or we have to compare the temperature of two
different bodies. Otherwise these scales of temperature do not show the
absolute value of thermal energy inside the body i.e. 0ºC temperature does not
mean that the body has zero value of thermal energy or zero intensity of heat.
The absolute zero temperature shown on the Kelvin scale means the temperature
at which there is no heat intensity or thermal energy in the body. It is the
temperature at which a gas will not occupy any volume and it occurs at
-273.15°C. Thus, 0°C equals to 273.15 K.
Thus absolute zero temperature (0 K) is that temperature, below which the temperature of any substance cannot fall and the temperature measured above this zero is called absolute temperature given in K. One degree on Celsius scale and Kelvin scale is same.
Temperature in K = 273.15 + Temperature in °C
1.4.2 Pressure (P)
It is defined as the force per unit area exerted by a body/matter/system on its surface in a direction normal to the surface. When a force is exerted on a fluid this force is transmitted throughout the fluid and is exerted in all direction with equal intensity. It is measured by pressure gauge which shows the pressure above the atmospheric pressure because atmospheric pressure always acts on the pressure gauge when it shows zero on the pressure scale. And any value shown on the scale is always the pressure above atmospheric pressure, which is called gauge pressure.
Thus Absolute pressure = Atmospheric Pressure + Gauge Pressure.
Or Pabs = Patm + Pg
For pressure below atmospheric, gauge pressure would be negative and is termed as vacuum.
Thus, Pabs = Patm - Vacuum.
In SI system of units the units of pressure is Pascal (Pa) such that
1 Pa = 1 N/m2
1 bar = 105 N/m2
Pressure can also be measured in terms of height of column of a liquid. For example, the pressure exerted by a height ‘h’ of column of a liquid of density ‘ρ’ filled in a tube/pipe over the area A of the bottom end of the tube will be weight of liquid column upon area, A, if top end of tube is closed. In this case pressure can be calculated by knowing the weight of liquid and area A of tube:
![]()
As the value of density, ρ for a given liquid is constant and ‘g’ is also an absolute constant, the pressure can indirectly be indicated in terms of height of column of a specified liquid. One commonly used unit of pressure in terms of height of mercury (Hg) column is given as:
1 mm of Hg = 1 Torr = 0.001 m of Hg
= 0.001× 13600 × 9.81 = 133.41 Pa
Standard Atmospheric Pressure = 760 mm of Hg
= 760 × 133.41 Pa
= 101300 Pa
= 1.013bar
1.4.3 Absolute volume (V)
It is defined as three dimensional space occupied by matter i.e. gas or liquid. It is measured in m3.
1.4.3.1 Specific volume (v)
It is defined as the volume occupied per unit mass of a gas/ fluid. It is reciprocal of the density. Unit of sp. vol. in S.I. system of units is m3/kg.