Module 1. Basic concept of thermodynamics
Lesson 2
THERMODYNAMIC CYCLE & ENERGY OF THERMODYNAMIC SYSTEM
2.1 State of a
System
It is the condition of a system at any particular moment which can be defined by a set of properties e.g. pressure, temperature, volume etc. The system may remain in a constant state under equilibrium position e.g. a gas filled in a cylinder of volume 1 m3 at pressure 10 bar and at atmospheric temperature is at a state defined by values of its pressure, volume and temperature. A state is represented by a point on the property charts like Pressure-Volume (P-V), and Temperature-Entropy (T-S) diagrams etc. Point 1 and 2 as shown in fig 2.1 represent state of gas.
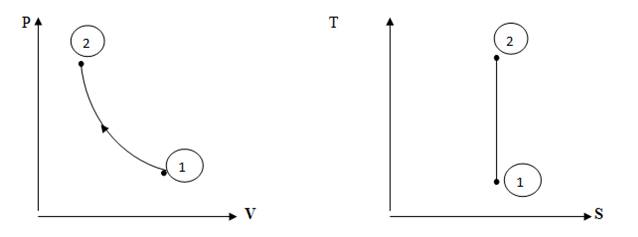
Fig. 2.1 Non-flow Isentropic Compression Process in a closed system
2.2 Thermodynamic Process
The continuous series of states followed by a working medium as it liberates, absorbs or transforms energy is defined as thermodynamic process. A thermodynamic process has some initial state and then a final state. During this process, the system may exchange the energy with the surroundings either in the form of heat or work or both e.g. if a gas, initially present at a state of 5 bar pressure and 2 m3 volume is compressed to a final state of 1 m3 volume and 12 bar pressure, it undergoes a series of states collectively named as a compression process. A process is represented by a straight or curved line on the property charts of the working medium i.e. the gas being compressed e.g. curve 1-2 indicates an isentropic compression process on pressure volume and temperature-entropy charts as shown in fig 2.1.
2.2.1 Point or path function
From all the above discussions one more consequence is that the change in value of a property of a system depends only on end states of the process and not on path of process e.g. a gas being compressed from 2 m3 to 1 m3 undergoes a change in volume by 1 m3 irrespective of the states in between the end states of this compression process. Thus property of system is always a point function. On the other hand some quantities like heat/work exchange depend on the path or process followed by the system and so these are path functions.
2.3 Thermodynamic Cycle
When a gas present at some initial state undergoes a series of processes and finally it returns back to same initial state then it is said to have completed a cycle. Thus a thermodynamic cycle means a series of processes, as shown on the P-V diagram below, such that the initial state of first process is same as the final state of last process. A thermodynamic cycle 1-2-3-4 is as shown in fig 2.2

Fig. 2.2 Thermodynamic cycle
2.4 Reversible & Irreversible Processes
A reversible process also known as quasi-static process is one which can be reversed at any stage to the same initial condition and also leaving no effect on the surroundings.
That means after completely reversing the process, the system and surroundings are exactly restored to ‘their’ initial condition. Hence a reversible process has following characteristics:
1) It must pass through the same states on the reversed path as were initially visited on the forward path.
2) This process when undergoes will leave no history of events in the surroundings.
3) It must pass through a continuous series of equilibrium states.
No real process is truly reversible but some processes may approach reversibility to close approximation.
An irreversible process is one which cannot be reversed or which is not reversible e.g. heat transferred through a finite temperature difference or work done on a gas enclosed in cylinder piston arrangement etc.
2.5 Reversible & Irreversible Cycles
A cycle containing all the reversible processes is reversible cycle.
A cycle containing one or more irreversible process is known as irreversible cycle.
In reality, a reversible cycle is not possible and is only of theoretical importance. In real practice, every thermodynamic device is based on any irreversible cycle.
2.6 Zeroth Law of Thermodynamics
Temperature Equality and Inequality: When two bodies or system come in contact with each other and if their thermal equilibrium is disturbed i.e. there is some change in properties then it is said that they have unequal temperature or temperature inequality. But after a substantial long time the change in properties is no more and the systems are again in thermal equilibrium then it can be said that now they have equal temperature i.e. temperature equality.
On this concept of temperature equality & inequality & thermal equilibrium, “Zeroth law of Thermodynamics” states that when two systems ‘A’ and ‘B’ have thermal equilibrium separately with a third system ‘C’, then ‘A’ and ‘B’ also must be in thermal equilibrium with each other. It is clearly shown in figure 2.3
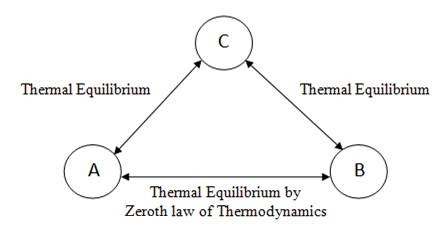
Fig 2.3 Zeroth Law of Thermodynamics
2.7 Energy, Work and Heat
Energy is a general term and in a very simple way, it is defined as the capacity of an agent to do work. In general we feel energy when it is in transition i.e. being transferred from one medium to other medium. E.g. heat transferred from one body to other body or work done on a gas to compress it to a lower volume or moving of wheels of car by its engine etc. The other phase of energy is stored energy which cannot be visible or felt e.g. chemical energy of fossil fuel, potential energy of a body placed at some height etc. In this way broadly, energy can be classified in two forms as (i) Transit energy and (ii) Stored energy, which can transform into each other.
In thermodynamics, we deal with both types of energies possessed or transformed by the working substance and their types are as given below:
(i) Stored Energy – Internal Energy
(ii) Transit Energy – Heat & work
2.7.1 Internal energy (u)
Internal energy is that energy which remains stored in the working substance in the form of thermal energy, which is due to molecular activity or lattice vibrations etc. and it is measured or indicated by the temperature of substance. Internal energy is directly proportional to temperature of substance, which is also called Joule’s Law. So it is a quantity depending on the state of the system.
U α T
ΔU α ΔT
ΔU = m Cv ΔT
Where, ΔU = Change in internal energy in Joules of the working medium in the system due to change in its temperature, ΔT
m = Mass of the working medium in kg
Cv = Specific heat at constant volume of the medium in J/ kg.K
2.7.2 Heat energy (Q)
It actually means thermal energy in transit i.e. heat exchanged by the system with its surroundings. The word ‘heat’ alone is seldom used and commonly used term is heat exchanged or heat absorbed or heat liberated etc. This exchange of heat between system and surroundings may be by any mode of heat transfer i.e. conduction, convection or radiation. Its intensity is directly proportional to difference in temperature of system and surrounding. The heat absorbed by the system is taken as positive and heat liberated by the system is taken as negative. It is a quantity, which depends on the process or path followed by the system.
2.7.3 Work energy (W)
It is the energy in transit
and occurs when a body is moving some distance under some force.
Mathematically, it is force multiplied by displacement. In thermodynamics, work
is there when there is some displacement on the boundaries of the system. When
the boundaries of the system expand i.e. displace the surroundings, the work is
taken as positive and when by some external pressure the boundaries of the
system contracts, the work is taken as negative. Thus positive work means
system is producing or doing work and negative work means work is being done on
the system by some external agency or surroundings. In real thermodynamic application,
mechanical work is exchanged through a rotating shaft crossing the boundary of
system. It is also a quantity depending on the process or path followed
by the system.
Units: In MKS system of units, unit of heat is Calorie (Cal) which is defined as amount of heat (thermal energy) required in raising the temperature of 1 gram of water by 1°C. And unit of work is kgf-m, which occurs, when one kg force moves a body by a distance of one meter. But in SI system of units, the units of heat and work are not different as both are different form of energies and also interchangeable. Here unit of heat is taken same as unit of work and it is Joule. One Joule (J) = One Newton x Meter (N-m)
Conversion factor of heat energy to work energy as given by Joules (from MKS to SI) is 1 Cal = 4.187 J.
2.8 Other Forms of Energy
2.8.1 Potential energy (P.E.)
It is due to position of some mass and is given by “m.g.h” where, m is the mass of the body having P.E., g is the acceleration due to gravity and h is the height of body reference to datum.
2.8.2 Kinetic energy (KE)
It is due to motion of mass and is equal to, ½ mv2 where m is the mass of the moving body/ matter and v is its velocity.
2.8.3 Flow energy (FE)
It is due to flow of mass across the boundary of system e.g. in open system, where mass continuously flow in & out of system, the energy contained by that mass also flows in or out of the system. If V is the volume flow rate of the medium (fluid/gas/vapor) at pressure P, then flow energy is given by ‘P.V’. It is also a form of work as:
P.V = P (A.L)
= (P.A).L
= F.l (Force × Distance)
In actual this energy is a kind of stored energy of gases/vapors indicated by a certain volume of gas at certain pressure. But as the gases flow their energies also flow.