Module 4. Microbiological methods of milk testing
Lesson 18
STANDARD PLATE COUNT
18.1 Introduction
The standard plate count (SPC) is suitable for estimating bacterial populations in most types of dairy products, and it is a reference method specified in the Grade A Pasteurized Milk Ordinance to be used to examine raw and pasteurized milk. This procedure is also recommended for application in detecting sources of contamination by testing line-samples taken at successive stages in the processing.
18.2 Principle
The test employs aserial dilution technique for easy quantification of the micro-organisms. The appropriate dilutions of the milk sample are mixed with a sterile nutrient medium that can support the growth of the micro-organisms, when incubated at a suitable temperature. Each bacterial colony that develops on the plate is presumed to have grown from one bacterium or clump of bacteria in the inoculums. The total number of colonies counted on the plates multiplied by the dilution factor to represent the number of viable micro-organisms present in the sample tested.
18.3 Procedure
18.3.1 Sampling
The sample is drawn aseptically using standard procedures.
18.3.2 Equipment and supplies
· Autoclave
· Balance
· Colony counter
· Dilution bottle
· Incubator
· Microwave oven
· Petri dish container
· Petri dishes
· Pipettes or Pipette aids
· Refrigerator
· Sterilizing oven
· Thermometer
· Water bath etc.
18.3.3 Materials
· Nutrient agar/ Tryptone dextrose agar
· Dilution water: Use only phosphate water for dilution
· Dilution water blanks: Fill dilution bottles with phosphate water so that each bottle will contain 99 or 9 ml (test tube) andautoclave at 121°C for 15 to 30 min.
18.3.4 Samples
Process the samples as soon as possible, but if necessary store the samples at 0° to 4.4°C until tested. Samples must be tested within 36 hours after the initial collection, and the time of plating must be recorded. Fluid milk samples that have been frozen should not be tested microbiologically, because freezing causes a significant change in the viable bacterial count in milk and hence, may give enormous results.
18.3.5 Sample preparation
· Mark each plate with sample number, dilution, and other desired information before making dilutions.
· Before opening a sample container, remove from the closure all obvious materials that may contaminate the sample. If desired, wipe the tops of unopened sample containers with a sterile cloth or paper towel saturated with 70% ethyl alcohol. Invert filled retail containers that contain air space 25 times, or until the contents are homogeneous. To ensure a homogeneous sample, where no air space is present, aseptically open the container and pour the product from the filled carton into a sterile container. The interval between mixing and removing the test aliquot must not exceed 3 min. Immediately before transferring the test portions of milk and of dilutions thereof, shake the container, making around 20 complete up-and-down (or back-and-forth) movements of about 30 cm in 7 s. Mechanical shaker may be used, if desired, to shake the dilution blanks uniformly for 15 s.
18.3.6 Dilution of samples
· For SPCs, select dilution(s) so that the total number of colonies on a plate is between 30 and 300. For example, where an SPC is expected to reach a number 5000, prepare plates containing 10-2 dilutions.
· Use a sterile pipette for initial and subsequent transfers from the same container, if the pipette is not contaminated. If the pipette becomes contaminated before transfers are completed, replace it with another sterile pipette. Do not flame to decontaminate. Use a separate sterile pipette for transfers from each different dilution.
18.3.7 Precautions to be taken
· Do not prepare or dispense dilutions or pour plates in direct sunlight. When removing a sterile pipette from the pipette container, do not drag the pipette tip over the exposed exteriors of the pipettes remaining in case because the exposed ends of such pipettes are subject to contamination. Do not wipe or drag the pipetteacross the lips and necks of vials or dilution bottles. Do not insert the pipette more than 2.5 cm below the surface of the sample or dilution. Draw test portions above the pipette graduation; then raise the pipette tip above the liquid level and adjust to the desired mark by allowing the lower side of the pipette tip to touch the inside of the container so that drainage is complete and excess liquid does not adhere when the pipette is removed from the sample or dilution bottle. Do not flame sterile pipettes.
· When delivering a diluted sample of a dairy product, hold the pipette at an angle of about 45° while the tip is touching the inside bottom of a Petri dish or the inside neck of a dilution bottle. Lift the cover of the Petri dish just high enough to insert the pipette. Deposit the sample away from the center of the dish to aid in mixing the sample with medium. Allow 2 to 4 s for the diluted milk or cream to drain from the graduation mark to the rest point in the pipette tip; then, holding the pipette in a vertical position, touch its tip once against a dry spot on Petri dish or on inside of the dilution bottle neck. Do not blowout. When 0.1 ml quantities are measured, hold the pipette as directed and let the diluted sample drain to the proper graduation point but do not retouch the pipette to the plate. After depositing the test portions in each series of plates, pour the medium.
18.3.8 Plating
· Melt the required amount of medium quickly in boiling water, in a microwave oven, or by exposing it to flowing steam in a partially closed container, but avoid prolonged exposure to unnecessarily high temperatures during and after melting. Discard melted nutrient agar or tryptone dextrose agar that develops a precipitate. Do not melt more medium than will be used in 3 hours. Do not re-sterilize the medium.Cool the melted medium promptly to approximately 45°C and hold it in a water bath between 44 and 46°C. Place the bulb of a thermometer in the medium or an aqueous solution of 1.5% agar in a separate container identical to that used for the medium; this temperaturecontrolled solution must have been exposed to the same heating and cooling as the medium. Do not depend on the sense of touch to indicate the proper temperature of the medium when pouring agar.
· Select the number of samples to be plated in anyone series so that not more than 20 minelapse between diluting the first sample and pouring the last plate in the Petri dish.
Wipe water from the outside of the medium bottles before pouring. Introduce 12 to 15 ml of liquefied medium at 44 to 46°C into each plate by gently lifting the cover of the Petri dish high enough to pour the medium. Carefully avoid spilling the medium on the outside of the container or on the inside of the plate lid when pouring. As each plate is poured, thoroughly mix the medium with the test portions in the Petri dish by rotating the dish first in one direction and then in the opposite direction, rotating and tilting the dish by hand or using mechanical rotators. Take care not to splash the mixture over the edge. Having thus spread the mixture evenly over the bottom of the plate, allow it to solidify on a plain surface. After solidification, invert the plates to prevent spreading colonies from developing because of accumulated moisture, and place the plates in the incubator.
18.3.9 Sterility controls of medium, dilutions, and lab wares
Check sterility of dilution water, medium, pipette, and Petri dishes by pouring control plates for each sterilization lot.
18.3.10 Incubating
Incubate plates at 32±1°C or 37°C for 48±3 h for SPC. Plates must reach the temperature of incubation within 2 h. Avoid excessive humidity in incubator to reduce the tendency toward spreader formation, but prevent excessive drying of the medium by controlling ventilation and air circulation. Agar in plates should not lose more than 15% of its weight during 48 h of incubation.
18.3.11 Counting of colonies on agar plates
Count the plates after the desired incubation period. Record the dilutions used and number of colonies counted on each plate. If it is impossible to count at once, after the required incubation store the plates at 0 to 4.4°C for not more than 24 h. For each lot of samples, record the results of sterility tests on materials used when pouring plates and the incubation temperature used.
Count colonies with the aid of magnification under uniform and properly controlled artificial illumination. Plates should be examined in subdued light. Routinely use a colony counter equipped with a guide plate ruled in square centimeters. Avoid mistaking particles of un-dissolved medium or sample, or precipitated matter in plates, for pinpoint colonies. Examine doubtful objects carefully, using higher magnification where required, to distinguish colonies from foreign matter.
a. Count all colonies, including those of pinpoint size, on selected plate. Record the dilution used and the total number of colonies counted.
b. Do not record counts on crowded plates from the highest dilution as too numerous to count. If the number of colonies per plate exceeds 300, count colonies in those portions of the plate that are representative of colony distribution, and calculate an estimated SPC from these counts. If there are fewer than 10 colonies per square centimeter, count colonies in 12 squares; select, if representative, 6 consecutive squares horizontally across the plate and 6 consecutive squares at right angles, being careful not to count a square more than once. When there are more than 10 colonies per square centimeter, count colonies in four such representative portions. In both instances, multiply the average number of colonies found per square centimeter by the area of the plate used to determine the estimated number of colonies per plate. Each laboratory must determine the area in square centimeters of the plates in use.
c. Spreading colonies are usually of three distinct types. The first type is a chain of colonies, not too distinctly separated, that appears to be caused by the disintegration of a bacterial clump. The second type develops in film of water between the agar and bottom of the dish. The third type forms in the film of water at the edge or on the surface of agar.
Automated colony counters, when determined in individual laboratories to yield counts that 90% of the time are within 10% of those obtained manually may be used for counting plates. When using colony-counters take the following precautions:
a. Align the Petri dish carefully on colony-counter stage.
b. Avoid ‘counting’ the stacking ribs or legs of plastic Petri dishes.
c. Do not count plates having unsmooth agar surfaces.
d. Avoid plates having food particles or air bubbles in the agar.
e. Do not count plates having spreaders.
f. Avoid scratched plates.
g. Wipe fingerprints and films off the Petri dish bottom before counting.
18.3.12 Calculating and recording of microbial counts
When calculating the SPC, report only the first two significant digits to avoid creating a fictitious impression of precision and accuracy. When making the conversion to the SPC, round-off to two significant figures by raising the second digit to the next highest number. Use zeroes for each successive digit toward the right from the second digit.
· Plates with 30 to 300 colonies:
a. Calculate the SPC as follows: N = ∑C/[(l x n1) + (0.1 x n2)]d.
Where,N = number of colonies per milliliter or gram of product,
∑C = sum of all colonies on all plates counted,
n1= number of plates in lower dilution counted,
n2 = number of plates in next higher dilution counted,
d = dilution from which the first counts were obtained.
b. When counts of duplicate plates fall both within and outside the 30 to 300 colony range, use only those counts that fall within this range.
· When plates from both dilutions yield fewer than 30 colonies each, record the count as less than 30 times d/l, where d/lis the reciprocal of the dilution factor.
· When plates from both dilutions yield more than 300 colonies each, estimate counts from the plates nearest 300 and multiply by the reciprocal of the dilution
18.3.13 Interpretingmicrobial counts
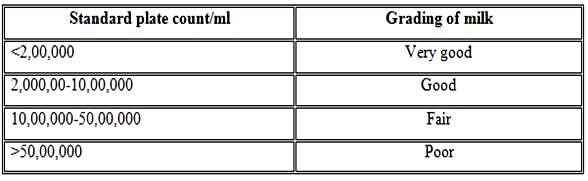
The quality of milk is adjusted by using the following details as shown in Table 18.1
Table 18.1 Grading of milk based on standard plate count test (BIS Standards)
18.3.14 Pasteurized milk
A standard plate count of lower than 30,000 cfu per ml. of pasteurized milk is indicative of satisfactory quality.
18.4 Advantages of SPC
· Enumeration of only viable microbes.
· Cultural and morphological differentiation based on colony characteristics.
· Suitable for determination of quality of milk samples like pasteurized milk and high grade raw milk with low bacterial number.
· Useful for pasteurized and for line testing at various stages of processing.
18.5 Disadvantages of SPC
· Gives only a rough estimate of microbial counts hence, not very accurate.
· Time consuming, laborious and cumbersome.
· Requires huge quantities of reagents, chemicals and glassware.
· Not a rapid method; at least 24 hours are required to get result.
· Not suitable for growth of all the species of bacteria present in milk.
· Temperature of incubation may not be optimum for the growth of all types of bacteria.
· Amount of sample taken may not be representative.
· Pathogens are not detected, because certain microbes like Mycobacterium tuberculosis cannot grow easily.
· Specific information regarding the type of micro flora is not obtained.