Module 3. Propagation of starter cultures
Lesson 4
MECHANICALLY AND CHEMICALLY PROTECTED SYSTEMS FOR STARTER PROPAGATION
4.1 Introduction
Starter cultures are generally propagated by using traditional method which is a cumbersome process involving several steps. Always a technical person is required to propagate the active and pure culture without any contamination. To avoid starter contamination and variation in activity of cultures, now-a- days, several methods have come into practice as detailed below.
4.2 Production Systems for Bulk Starter Cultures
The aim of production systems used for bulk starter cultures is to produce a pure active culture free from contamination, mainly from bacteriophage.
1. Simple microbiological technique
2. Employment of mechanically protected equipment
3. Propagation in Phage Resistant Medium /Phage Inhibitory Medium (PRM/PIM)
4.3 Mechanically and Chemically Protected Systems
4.3.1 Employment of mechanically protected equipment
Two aspects are important, when employing mechanically protected equipment i.e.,
4.3.1.1 Lewis system
This system
involves the use of a two way hypodermic needle to carry out the transfer of
stock cultures to mother culture, mother to feeder and feeder to bulk starter.
All inoculations take place through a barrier of chlorinated water. Re-usable,
collapsible polythene bottles are used at each stage. The bottles are fitted
with as tell rubber seals and a screw cap.
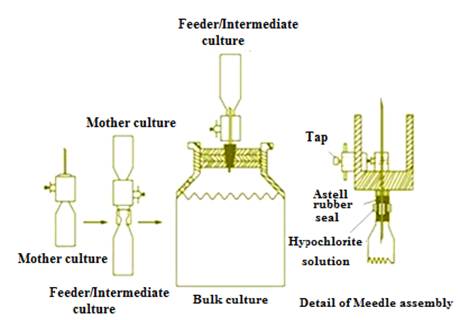
Fig. 4.1 Schematic illustration of the Lewis system for starter culture transfer
4.3.1.2 Alfa-laval system
The principle is similar to the above but the tank is of different design. Sterilized air is used instead of squeezing. Two needles are used. Short needle are used for sending sterilized air in and to force the culture through long needle to next container.
The other systems are- Terlet-Zutphen system, Jones system, Silkberg /Pasialc system etc.
4.3.2 Propagation in phage resistant medium /phage inhibitory medium (PRM/PIM)
Phage resistant medium is used mostly for mesophilic starter cultures by using the ingredients like milk solids, sugar, stimulatory compounds (yeast extract, pancreatic extract), phosphate citrate buffer, chelating agents (ammonium or sodium phosphate). These chelating agents bind with Ca++ and Mg++ as these ions are required by the bacteriophage during the proliferation and replication. The bacteriophage also requires these ions for the phage adsorption onto the bacterial cell.
4.4 Starter Systems
4.4.1 Bulk set
The supplier provides the user with a small amount of culture which is then fermented by the user through one or two stages to produce a volume of bulk starter. Typically fermentation takes place at 22-26°C for 14-16 hr for mesophilic and at 43°C for 4.5 to 5 hr for thermophilic starter. A significant portion of this time is taken up by the lag phase as cells repair themselves from their storage mode. This starter then may be used immediately by inoculating into production milk (1% for cheddar, 3% for yoghurt).
4.4.2 Direct vat inoculation
This is made available in more concentrated form in large volumes such that the product can be inoculated directly into the production milk. In both cases inoculation into pasteurized medium ensures an active starter dominating the fermentation.
4.5 Main Operating Methods
The main starter operating systems for controlling the bacteriophages are
a) Rotational
b) Non-rotational
c) Dutch P/L system
4.5.1 Rotational
This is the method of choice traditionally. Different culture is used for each fill of the vat and the same culture would not be reintroduced for 5 days or for one production week. The concept is that the regular change of a culture would prevent the build-up of a bacteriophage and thus avoid starter failure because phages are generally but not exclusively virulent against one host type. The effectiveness of the system can be improved by regular phage monitoring to highlight problematic cultures allowing them to be removed from use before they fail.
4.5.2 Non-rotational
This is originally developed in New Zealand. This is based on the identification of a small number of highly characterized single strain phage resistant bacteria. These individual strains are introduced into the plant normally as blend of three, where they are used continuously. Phage testing to monitor the development of phage against any of the strains is carried out. When the phage levels are likely to affect acid production (105 or above are detected) the strain is removed and replaced by a suitable alternative. It is normal for such systems to require very great attention to hygiene and production discipline. Such systems have been attended by outstanding success particularly in their ability to practically eliminate bacteriophage problems. However, staff knowledge and plant design must be compatible with the hygienic standards.
4.5.3 Dutch P/L system
It is based on the proposition that if mixed cultures are transferred in an environment which deliberately exposes them to bacteriophage and the resulting mix of cultures may include new phage resistant mutants and this will help operate satisfactorily in the production environment. But it is heavily dependent on having skilled microbiologists on site to manage the system.
4.6 Some Points for an Effective Starter System
- Define
the starter objectives in the plant.
- Recognize
the physical as well as human constraints.
- Ensure all staff involved understands the system
and what is required of them.
- Ensure all
staff involved understands the bacteriophage, its seriousness and the part
they can play in limiting its effects.
- Ensure
that the movements of air, water, people and product are known and their
role as potential channels for phage attack.
- Consider
the role of HACCP.
- Be
obsessive in hygiene and production disciplines associated with starter
production and usage.
- Institute prospective phage monitoring by daily
whey testing.
- Monitor the results against the objectives defined.