Module 4. Metabolism of starters and biochemistry of fermentation
Lesson 5
METABOLISM IN STARTER CULTURES
5.1 Introduction
The primary function of Lactic Acid Bacteria (LAB) is to produce lactic acid by fermenting the milk sugar lactose. Fermentation of lactose is carried out by different pathways by different types of starter cultures. Microbial cells derive their energy requirements via
i. Fermentations
ii. Tri-carboxylic acid cycle
iii. Cytochrome system for terminal electron transport
iv. Enzymes that operate anaplerotic pathway
5.2 Production of Lactic Acid (Carbohydrates Metabolism)
Lactose is a disaccharide constituting about 40% of milk solids. Lactic acid bacteria use two strategies to hydrolyse lactose.
1. β-D galactosidase ( lactase or β- gal)
2. β-D phosphogalactosidase (β- p-gal)
Many lactic acid bacteria possess both β- gal and β- p-gal enzymes. Most lactobacilli have β- gal enzyme except L. casei which exhibits β- p-gal activity. Lactococci in natural systems posses both but in starter cultures only β- p-gal activity is seen.
Lactose is transported into the lactic acid bacteria via two systems i.e. through Permeases and through Phospho-enol-pyruvate dependent phospho-transferase system (PEP: PTS). Lactose is broken down to glucose and galactose by β-D galactosidase by catalization of 1-4 β- galactosidic bond in lactose.
Glucose is used in both aerobic and anaerobic organisms. Aerobic organisms are evolved from anaerobes and retained the fermentative pathway, but being able to utilize oxygen, the aerobes have mechanisms to complete the catabolism of end products to CO2 and H2O. Anaerobic pathway leads to incomplete breakdown of glucose releasing small amounts of energy. TCA cycle is aerobic and in this glucose or acetate is oxidized to CO2 and H2O. Electrons removed from substrates, as they get oxidized, are transported to molecular oxygen.
5.2.1 Homo-fermentation
In homo-fermentative lactic acid bacteria the lactose transport across the cell membrane involves the Phospho-enol-pyruvate dependent phospho-transferase system wherein the lactose is phosphorylated to lactose-P (glycosyl β galactoside-6-Phosphate) during its translocation (Figure 5.1).
In this system the lactose-P is hydrolyzed by β-phospho-galactosidase (β-D phosphogalactoside- galactohydrolyse) to D-glucose and galactose-6 phosphate. Glucose is metabolized to pyruvate via Embden Meyerhof Pathway (EMP). In the metabolism of galactose it is first converted to glyceraldehydes -3 phosphate via D-tagatose-6 phosphate pathway.
So two pathways are involved
1. EMP for the metabolism of glucose
2. Tagatose 6-phophate pathway for metabolism of galactose
Lactose in organisms
like S. thermophilus and Lb. bulgaricus is transported into the cell by the
enzyme Permease and or PEP: PTS system. These
organisms possess β-D galactosidase (β-gal)
enzyme which hydrolyses lactose into β-D galactose
and D-glucose. Glucose is converted into lactic acid via EM (Embden Meyerhof) pathway. Galactose
is excreted from the cell or may be utilized by other bacteria producing other
than lactic acid. But some of the homofermentative
organisms under some special conditions such as absence or limiting glucose or
lactose or particular pH under take heterofermetations.

Fig. 5.1 Homo fermentation
5.2.2 Hetero fermentation
Lactic acid bacteria producing large quantities of ethanol, co2 as well as lactic acid when grown on lactose or glucose are considered as hetero fermentative organisms. The end products of CO2, lactic acid and ethanol during heterofermetation are formed through three distinctive chemical reactions as detailed below and as depicted in Figure 5.2.
· CO2 is produced by the oxidative decarboxylation of 6-phosphogluconate
· Ethanol is produced by reduction of acetaldehyde
·
Lactic acid
is produced by EM pathway
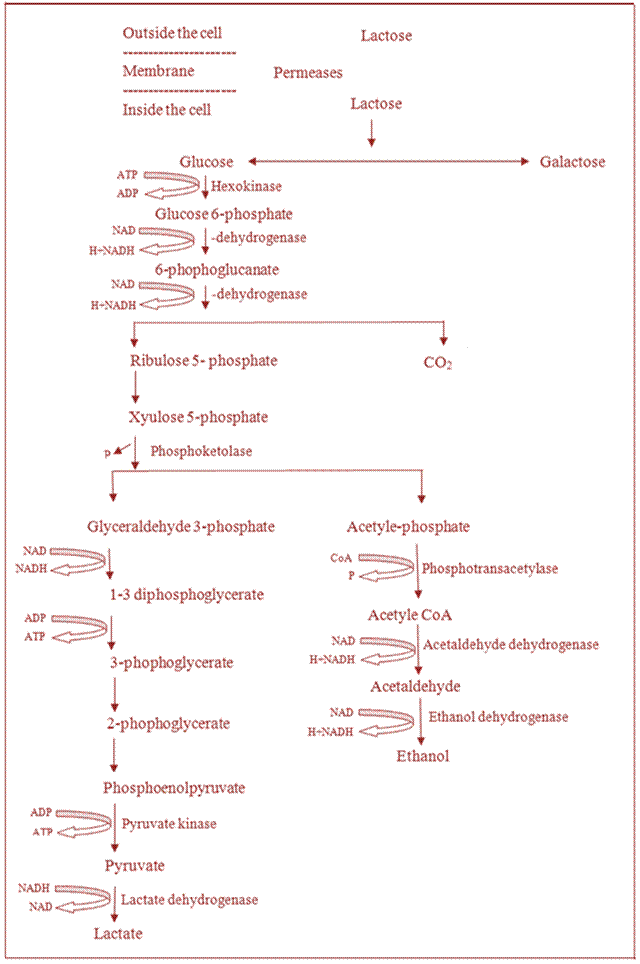
Fig. 5.2 Hetero fermentation
5.3 Production of Flavour Compounds (Citrate Metabolism)
Citrate content of milk varies
between 1.4 – 2.0 g per kg of milk. Cellular uptake of citrate is by plasmid
encoded enzyme citrate Permease in Lactococcus lactis ssp lactis biovar diacetylactis.
Citrate lyase is induced by the presence of citrate.
There are two pathways for the formation of diacetyl,
as shown in Figure 5.3.

Fig. 5.3 Citric acid metabolism
1. Chemical—by oxidative decarboxylation of α acetolactate which is excreted into milk by bacteria cells.
2. Enzymatic—diacetyl is formed in cells by the reactions of acetyl Co-A and activated acetaldehyde
Diacetyl is an intermediate product. So concentration varies during manufacturing of cultured dairy products and it may disappear subsequently. There is relationship between the concentration of diacetyl and acidity of the product. To achieve the highest concentration of diacetyl in fermented milk the following criteria are to be fulfilled i.e pH has to ≤ 4.6 for controlling diacetyl and the citric acid has to be completely fermented.
Dairy products require some diacetyl concentration for acceptable flavour. Active diacetyl reductase reduces flavour by converting diacetyl to acetoin. The potential source of the diacetyl reductase is the contaminating bacteria especially Gram negative psychrotrophs, coliforms and yeasts.
5.4 Protein Metabolism
The hydrolysis of protein to yield amino acids can be accomplished in two major stages:
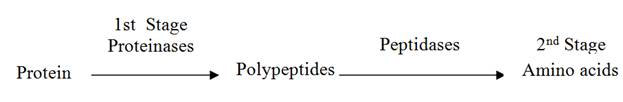
The range of products released by proteolysis is dependent on two main factors:
1. Firstly, the components of the milk protein fraction, and
2. Secondly, the types of proteolytic enzymes that the starter organisms may possess.
Proteolytic enzymes: These enzymes, as the name suggests, are specific in their action, and their main function is to catalyse the hydrolytic cleavage of the peptide bonds which form the backbone of the protein molecule.
Enzymes acting on peptide bonds are known as peptide hydrolases, and till date a large number of such enzymes have been identified. In the past the name given to an enzyme was derived from the substrate involved. At present it can be observed that the peptide hydrolases are divided into two main groups, i.e. the peptidases and the proteinases. The peptidases are also known as exopeptidases which specifically catalyse the hydrolysis of terminal α-amino or α-carboxyl groups of the peptide bonds
The proteinases are also known as proteolytic enzymes, endopeptidases or peptidyl-peptide hydrolases, which catalyse the hydrolysis of the internal peptide linkages of protein.
Yoghurt starter cultures are weak in proteolytic fermentation. S. thermophilus and L. bulgaricus may produce proteolytic enzymes, during the fermentation, which cause a significant degree of proteolysis, and this activity may be important for the following reasons:
(a) The enzymatic hydrolysis of milk proteins results in the liberation of peptides of varying sizes and free amino acids,
(b) The possible changes due to proteolytic activity can affect the physical structure of yoghurt.
(c) The liberation of amino acids into the milk is essential to the growth of S. thermophilus.
(d) Although amino acids and peptides may not contribute directly towards the flavour of yoghurt, they do act as precursors for the multitude of reactions which produce flavour compounds.