Module 3. Processing of fruits and vegetables
Lesson 9
CANNING AND OTHER METHODS OF THERMAL PROCESSING
9.1 Introduction
In continuation of the previous lesson, the reader would now be introduced to some of the other post harvest processing operations of fruits and vegetables such as canning, osmotic dehydration, etc.
9.2 Canning
The process of sealing fruits and vegetables or any other foodstuffs hermetically (air tight) in containers and sterilizing them by heat for long storage is known as canning. In 1904, Nicholas Appert of France invented this process and he is called as “Father of Canning”. The process of canning is also known as Appertization. Fruits and vegetables are canned in the season when the raw material is available in plenty. The canned products are sold in off-season and give better returns to the grower. The process flow diagram for canning is given in Fig. 9.1. Most of the preliminary operations of canning such as selection of the raw material, washing, sorting and grading, blanching have already been discussed in previous lesson and hence not repeated.
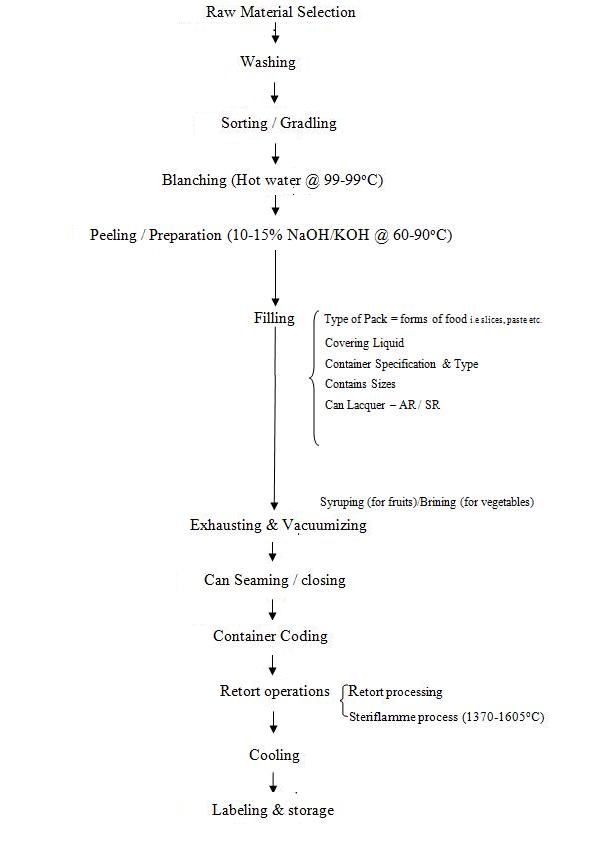
Fig. 9.1 Flow diagram for the canning process of fruits and vegetables
9.2.1 Can filling
Can filling is the process of aseptically filling whole or sized fruit or vegetables into the containers. The cans are washed with water or subjected to steam jet to remove any adhering dust or foreign matter. Tin cans made of thin steel plate of low carbon content, lightly coated on either side with tin metal to a thickness of about 0.25 mm are usually used in canning. The thickness of coating varies from 0.31 mm to 1.54 mm. The following are the different types of base plates used for can manufacture:
A) Type L: It is a high purity steel with low metalloid and residual content. This kind of base plate is used for highly acidic foods.
B) Type MR: It is a low metalloid steel with no severe restriction on residual content. It is used for moderate acid foods.
C) Type MC: It is similar to MR type but has high phosphorus content to give mechanical strength or stiffness. It is usually used for low acid foods.
9.2.1.1 Lacquering
It is difficult to coat steel plate uniformly with tin during the process of manufacture. Small microscopic spaces are always left uncoated, although the coating may appear perfect to the naked eye. The content of the can may react with the exposed parts of container and cause discolouration of the product or corrosion of the tin plate. When the corrosion is severe, the steel is attacked and black stains of iron sulphide are produced. Hence, it is necessary to coat the inside of the can with some material like lacquer, which would prevent discolouration, but would not impart its own flavor or injure the wholesomeness of the contents. The process of coating of inner side of the can to prevent discolouration of the product is called as lacquering. Lacquers include oleo-resinous material, synthetic resins, phenolic resins, epoxy resins and vinyl resins. There are two types of lacquers: (a) acid resistant and (b) sulphur resistant. The acid-resistant lacquer is ordinary gold coloured enamel and the cans treated with it are called as A.R-enamel cans. The sulphur-resistant lacquer is also of golden colour and the cans coated with it are called C-enamel cans or S.R. cans. Acid-resistant cans are used for packing of fruits of the acid group with soluble colouring matter such as raspberry, strawberry, red plum, coloured grapes, etc. Sulphur-resistant cans are used for non-acid products like peas, corn, beans, etc.
9.2.2 Syruping and brining
In canning, syrups are added to fruits whereas brine (salt solution) is added to the vegetables. Purpose of adding syrups or brine is to improve the flavor, fill the space between the pieces of canned product and aid in the heat transfer during sterilization. Cane sugar, glucose syrup, invert sugar and high fructose corn syrups are used for canning. Brine containing 1 to 2 percent of common salt is generally used for vegetables. Strength of syrup is measured by using hydrometer or a refractometer while strength of brine is measured by salometer or salinometer. The syrup or brine should be added to the can at a temperature of about 90°C, leaving suitable headspace in the can.
9.2.3 Exhausting
Exhausting usually means heating the can and can contents before sealing. Sometimes it is may also refer to the treatment of the container under a mechanically produced vacuum. But in either case it is done to remove air from the can interior and prevent corrosion. It also prevents undue strains upon the can during sterilization and prevents overfilling of can contents. Removing of air also helps in better retention of vitamins especially of vitamin C. The other advantages of the exhaust process are prevention of bulging of the can when stored at high altitudes or in hot climates. In heat exhaust method, the cans are generally passed through a tank of hot water at about 92-97°C or on a moving belt through a covered steam box. The time of exhaust varies between 5 to 25 minutes. After exhausting cans are immediately sealed with the help of double rolling operation of seamer.
9.2.4 Processing of the cans
The term “processing” as used in canning technology, means heating of canned foods (fruits, vegetables and other food stuffs) to inactivate bacteria. This is also called as “retorting”. Processing consists of determining just the temperature and the extent of cooking that would suffice to eliminate all possibilities of bacterial growth. In retort, saturated steam is supplied to heat the product. Time-temperature combination of processing depends upon the type and physical state of the product, the heat resistance of microorganisms or enzymes likely to be present in the food, the heating conditions, pH of the food, and size of the can to get complete sterility. In low acid foods (pH > 4.5), Clostridium botulinum is the most dangerous heat resistant spore forming pathogen likely to be present. Under anaerobic conditions inside a sealed can it can grow to produce a powerful exotoxin, botulin, which is sufficiently potent to be 65% fatal to humans. Because of the extreme hazard from botulin, the destruction of this microorganism is therefore a minimum requirement of heat processing (i.e. in canning and sterilization). Normally foods receive more than this minimum treatment as other more heat-resistant spoilage bacteria may also be present. In more acidic foods (pH 4.5 – 3.7), other microorganisms (e.g. yeast and fungi) or heat-resistant enzymes are used to establish processing times and temperatures. In acidic foods (pH < 3.7), enzyme inactivation is the main reason for processing and hence, heating conditions are less severe. The preservative effect of heat processing is due to the denaturation of proteins, which destroys enzyme activity and enzyme-controlled metabolism in microorganisms.
The rate of destruction is a first-order reaction; that is when food is heated to a temperature that is high enough to destroy contaminating microorganisms, the same percentage die in a given time interval regardless of the number present initially. This is known as the logarithmic order of death and is described by thermal death rate curve (Fig. 9.2). The time needed to destroy 90% of the microorganisms (to reduce their numbers by a factor of 10) is referred to as the decimal reduction time or D-value. D-values differ for different microbial species (Table 9.1) and a higher D-value indicates greater resistance. The thermal destruction of microorganisms is temperature dependent and cells die more rapidly at higher temperature. By collating D-values at different temperatures, a thermal death time (TDT) curve is constructed (Fig. 9.3).
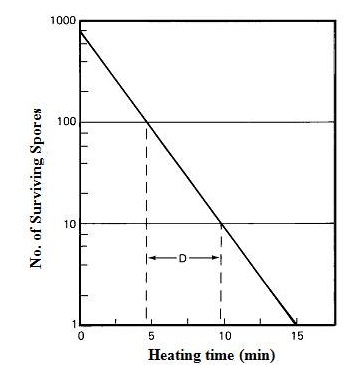
Fig. 9.2 Thermal death rate curve
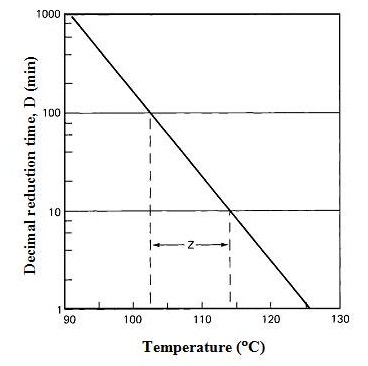
Fig. 9.3 Thermal death time curve
The thermal death time or F-value is used as a basis for comparing heat sterilization procedures. F-value is the time required to achieve a specified reduction in microbial numbers at a given temperature and it represents the total time-temperature combination received by a food. The slope of the TDT curve is termed the z-value and is defined as the number of degrees Celsius required to bring about a 10-fold change in decimal reduction time. F-value is quoted with suffixes indicating the retort temperature and the z value of the target microorganism. For example, a process operating at 110°C based on a microorganism with a z-value of 10°C would be expressed as F10110. Hence, D-value and z-value are used to characterize the heat resistance of a microorganism and its temperature dependence, respectively while F-value is used for comparing sterilizing procedures.
Table 9.1 Heat resistance of some spore-forming bacteria used as a basis for heat sterilization of low-acid foods
|
Microorganism |
z-value (°C) |
D121 value (min) |
Typical foods |
|
Thermophilic (35-55°C) |
|||
|
Bacillus stearothermophilus |
9 – 10 |
3.0 – 4.0 |
Vegetables, milk |
|
Clostridium thermosaccharolyticum |
7.2 – 10 |
3.0 – 4.0 |
Vegetables |
|
Mesophilic (10-40°C) |
|||
|
Clostridium sporogenes |
9.9 – 11.1 |
0.7 – 1.5 |
Meats |
|
Bacillus subtilis |
4.1 – 7.2 |
0.3 – 0.76 |
Milk products |
|
Clostridium botulinum toxins A and B |
5.5 |
0.1 – 0.3 |
Low-acid foods |
|
Bacillus coagulans |
6 – 9 |
0.01 – 0.07 |
Milk |
|
Bacillus cereus |
36 |
3.9 |
Milk |
|
Psychrophilic (-5 to -1.5°C) |
10 |
3.0 (60°C) |
Low-acid foods |
9.2.4.1 Rate of heat penetration in processing
Heat is transferred from steam or pressurized water through the container and into the fruit or vegetable or any other canned food. The heat transfer patterns in canned foods are given in Fig. 9.4. The rate of heat penetration is measured by placing a thermocouple at the thermal centre of a container (the point of slowest heating) to record temperatures in the food during processing (Fig. 9.5). It is assumed that all other points in the container receive more heat and are therefore adequately processed. The zone of slowest heating in a container is called as cold point, which is most difficult to sterilize. In cylindrical containers the thermal centre is at the geometric centre for conductive heating foods and approximately one third up from base of the container for convective heating foods (Fig.9.6).
The important factors that influence the rate of heat penetration into a food are given below:
· Type of product – Liquid or particulate foods (for example peas in brine) in which natural convection currents are established heat transfers faster than in solid food in which heat is transferred by conduction (for example pastes or purees). The low thermal conductivity of foods is a major limitation to heat transfer in conduction.
· Size of the container – Heat penetration to the centre is faster in small containers than in large containers.
· Agitation of the container – End-over-end agitation and to a lesser extent, axial agitation increases the effectiveness of natural convection currents and thereby increases the rate of heat penetration in viscous or semi-solid foods (for example beans in tomato sauce).
· Temperature of the retort – A higher temperature difference between the food and the heating medium causes faster heat penetration.
· Shape of the container – Tall containers promote convection currents in convective heating.
· Type of container – Heat penetration is faster through metal than through glass or plastics owing to differences in their thermal conductivity.
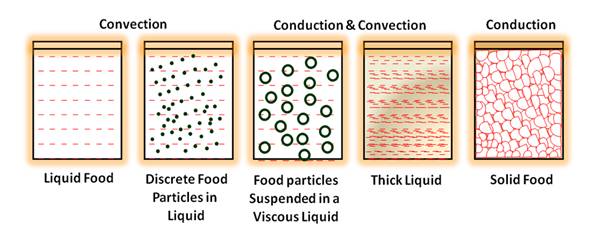
Fig. 9.4 Heat transfer pattern in canned foods
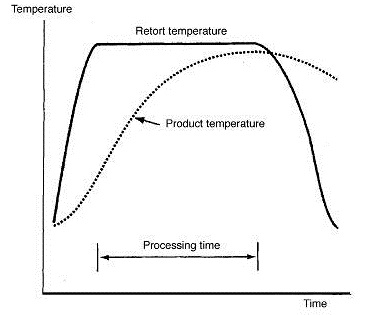
Fig. 9.5 Heat penetration into a can of conductive heating food
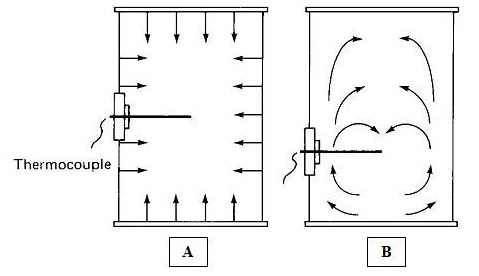
Fig. 9.6 Heat transfer into containers by conduction (A) and convection (B)
9.2.5 Cooling
Immediately after processing, cans are cooled to room temperature in cold water bath or water tank. Once cooling is carried out, the outer surface is dried and labeled.