Module 3. Processing of fruits and vegetables
Lesson 10
FREEZING: PRINCIPLE, METHODS AND APPLICATIONS
10.1 Introduction to Freezing
Freezing preservation is one of the most beneficial preservation methods. It involves, conversion of liquid content of food into ice crystals, which lowers down water activity and microbial growth is arrested due to cold shock. Pure water is frozen at 0°C but since fruits and vegetables contain number of dissolved solids like sugars, acids, they freeze at below 0°C.
10.2 Process of Freezing
During freezing the commodity cools down below their freezing point but don’t freeze this phenomenon is called as super cooling. It is shown by AB phase of curve (Fig. 10.1). At super cooled storage nuclei formation (nucleation) which is the first and most important step in ice-crystal formation in in freezing process. Here the temperature of water will be lower than 0°C but it will remain in liquid form. At this stage, further lowering of temperature result in the formation of ice crystals. The second step is called crystal growth stage. The release of heat of crystallization further enhances temperature (BC). Since food molecules contain substantial amount of soSlute hence, a progressive freezing occurs as depicted in Fig. 10.1. Various water molecules gathers around nuclei and due to subsequent addition, crystal growth occurs. Nucleation may be either due to chance orientation of molecule or due to induction of nuclei from outside, but in fruits & vegetable mostly chance nucleation occur. In next step, crystal growth around these nuclei occurs and as a result of ice-crystal formation, heat of crystallization is generated, which cause increase in temperature of commodity. This Tes is shown by BC lines. So, time taken by freezing curve from initial cooling to E point of curve is known as thermal arrest time. It determines how quick or slow freezing process is. After this point more ice crystal formation takes place and temperature lowers down.
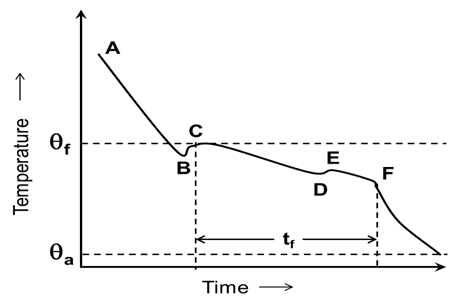
Fig. 10.1 Schematic diagram of freezing process
10.3 Advantages of Freezing
o No nutrient loss
o Retain freshness of commodity.
o Retain colour and flavor constituents.
o No microbial contamination.
o No respiration, hence longer shelf-life.
10.4 Effect of Freezing
Freezing process is divided into two broad categories viz. slow freezing and quick freezing.
· Slow freezing: when thermal arrest time is more than 30 min.
· Quick freezing: Thermal arrest time is less than 30 min.
In slow freezing, less number of nuclei is formed and as a result of slow freezing more concentrated solution is left in inter-cellular spaces which causes osmotic effect and liquid comes out from cells. This affects turgidity of cell and they collapse and on thawing cannot regain their original shape. Also, crystals forms are larger in size and pierce the cell membrane, puncture it and damage the cells. Whereas in quick freezing large numbers of nuclei are formed, hence having large numbers of crystals of smaller size evenly distributed within the cell and in the intercellular space. Since process is very quick, hence no concentration effect occur and commodities retain their original shape.
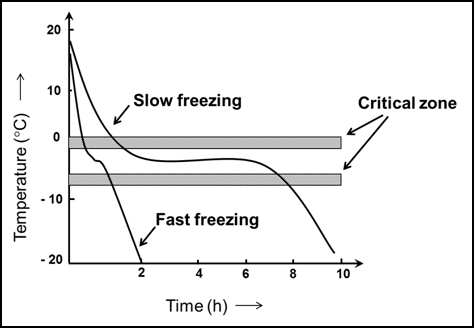
Fig. 10.2 Schematic diagram of temperature changes of food through the critical zone during freezing process
10.5 Freezing Methods
Mode of heat transfer in freezing food product is convection. Following points should be considered while selecting a freezing method:
a)
Product
dimension
b)
Shape
c)
Specific heat
d)
Thickness of
pieces
e)
Freezing rate
f)
Packaging
g)
Food product
components.
10.5.1 Air freezing
This is an oldest method of freezing and utilizes cool air having a temperature of -18 to -40°C as freezing method. Different types of air freezing are:
a)
Tunnel freezing
b)
Fluidized bed
Freezing
c)
Air blast
freezing
Air-blast freezers recirculate air over foods at between -30°C and -50°C at a velocity of 1.5-6.0 ms-1. The high air velocity reduces the thickness of boundary air films. Air flow is either parallel or perpendicular to the food and is ducted to pass evenly over all food pieces.
Air freezing may result in
· Excessive drying
· Costly
· More efficient & more rapid heat transfer
· Less product dehydration & less frequent requirement of frosting.
· Short freezing time so less moisture loss.
10.5.2 Plate freezing
Packaged or fresh commodities are placed over the surface of plate cooled by refrigerant in a cylindrical scraped – surface heat exchanges. Double plates are specially used in retail storage. Plate freezing is a slow freezing process and packages must be of uniform thickness.
10.5.3 Liquid immersion freezing
Certain liquids are used as refrigerant which are known as cryogens. Example: Liquid Nitrogen, Liquid NO2, Liquid Ammonia, etc.
10.5.4 Cryogenic freezing
Freezers of this type use a change of state in the refrigerant (or cryogenic) to absorb heat from the freezing food. The heat provides the latent heat of vaporization or sublimation of the cryogen. The cryogen is in intimate contact with the food and rapidly removes heat from all surfaces to produce high heat transfer coefficients and rapid freezing. The two most common refrigerants are liquid nitrogen and solid carbon dioxide. The main advantages of cryogenic freezing are as follows:
· Short freezing time due to high heat transfer
· Reduction in flavor loss
· Reduction in drip loss
· Reduction in oxidative changes
· Improved texture of the product
· Suitable for freeze sensitive products
The main disadvantage of cryogenic freezing is relatively high cost of cryogens.
10.6 Changes Associated With Ice Formation
10.6.1 Volume changes
The volume of ice is 9% greater than pure water when water is transformed into ice at 0°C, and hence upon freezing there is expansion of foods. However, few exceptions also exist. For example, highly concentrated sucrose solution. The degree of expansion depends upon (a) composition, (b) fraction of water that fails to freeze, and (c) temperature range.
· Moisture High moisture contents in foods produce greater changes in volume.
· Cell arrangement in fruits and vegetables Fruits and vegetables have intercellular air spaces which absorb internal increases in volume without large changes in their overall size.
· Concentration of solutes High concentrations reduce the freezing point and foods do not freeze or expand at commercial freezing temperatures.
· Freezer temperature It determines the amount of ice and hence the degree of expansion.
· Temperature range Observed changes depend upon the temperature range to which food product is exposed.
o Cooling of the specimen: contraction
o Ice formation: Expansion
o Cooling of ice-crystals: contraction
o Solute crystallization: contraction
· Crystallized components: These include ice, fats and solutes, contract when they are cooled and this reduces the volume of food.
10.6.2 Concentration of non-aqueous system
During freezing water freezes first. So nearly all the dissolved substances are therefore concentrated in the diminished quantity of water. So in some manner it is similar to dehydration. Unfrozen phases have changed physico-chemical properties like pH, titrable acidity, ionic strength, viscosity, freezing point, or potential. Dissolved gases will be removed/expelled from the space. Water structure & water-solute interaction may be drastically increased, macromolecules will come together and many detrimental reactions may occur. As freezing progresses, concentration of a particular solute increases and eventually reaches or exceeds their respective saturation concentration crystallization.