Module 11. Confectionery products
Lesson 39
CHEWING GUMS AND BUBBLE GUMS
39.1 Introduction
Chewing gum is a type of gum traditionally made of chicle, a natural latex obtained from the sapodilla tree (Manilkara chicle) and jelutong-pontianank, latex of jeluotong tree. Either of the latexes was considered as main components for making chewing gum base. However, for economical and quality reasons, modern confectioners use natural or synthetic rubber (polyisoprene) or hydrocolloids instead of chicle. Bubble gums are essentially chewing gums which are less sticky and stretch more easily. Different gum bases are used for chewing gum and bubble gum. Bubble gum base contains either higher levels of polymers or polymers of high molecular weight. Either of these makes the gum base more extensile and hence able to form bubbles. Special non-sticking gum bases have been developed in order to avoid the problem of discarded gum becoming nuisance.
39.2 Role of Ingredients Used in Chewing Gum
39.2.1 Hydrocolloids
Hydrocolloids are the key ingredients for gums manufacture. They are high molecular weight polymers of plant, animal or microbial origin that form viscous solutions or gels on addition of water. They gel and thicken but also stabilize by preventing syneresis, fixing flavours and inhibiting sugar crystallization, give transparency, brilliance, adhesion and ease foaming. Some of the hydrocolloids commonly used in confectionery are described below:
a. Agar agar: It is a seaweed and swells in water and exhibits a high gelling power at low (1-2%) concentrations to give a hard-brittle short-breaking bite with good clarity. It is one of the most potential gel-forming agents known. The strength is mainly proportional to the agarose rather than agaropectin content, which forms double helices on gelation. It has a unique property to form gels which only sets at 32-39°C yet does not melt until temperature reaches 85-90°C. This aspect is advantageous in allowing the incorporation of flavours, acids, colours, etc. at cooler temperatures prior to shaping and setting.
b. Bacterial gums: Xanthan gum from Xanthomonas campestris, gellan gum from the fermentation of Pseudomonas elodea, etc. are potential gums which find applications in confectionery. Gellan is functional at very low concentration (about 0.5%) to give hard-brittle short-textured gels. Setting temperature is 40-50°C yet melting temperature is 90-100°C.
c. Gelatin: Food grade gelatin is made by aqueous extraction of mammalian collagen by lime or acid treatments of skin, bovine or porcine raw materials. Religious reasons may prevent the use of porcine sources. The thermoreversibility of gelatin gels (at around 40°C) gives its main organoleptic feature of a smooth elastic texture which melts agreeably in the mouth. Commercial gelatin is available in leaf, sheet, granule and powder forms. In India, as per Food Safety and Standards (2011) Rules, food grade gelatin is permitted as an additive.
d. Gum acacia: It is a tree exudate, where it forms as ‘tears’ and also called as gum arabic. Each tree yields between 50 and 100 g of gum per year. It is available in lumps, powdered and as purified, standardized, spray-dried. It is the preferred hydrocolloid for many long-lasting, chewy, gum sweets and is used at levels from 10-60%. Resistance to melt away, shape stability, good clarity, bland taste and odour with minimal sweetness and pliable texture with low adhesion during consumption are the key factors of this gum. Its viscosity reduces greatly with increased temperature, making it suitable for fluid-deposited goods.
e. Pectin: It is a polymer of galacturonic acid obtained mainly from apple or citrus fruits. Pectins are characterized by their degree of esterification or methoxylation and gel strength. It forms gels at 0.5-4.0% concentration and have a delicate fruit-like short texture and mouth feel with very good flavour release. Low-methoxy pectin does not require acid and a good gel can be obtained by diffusing calcium ions into the system.
f. Modified starches: They are the starches modified by chemical reaction or physical means in order to adapt it for a specific application or improve its stability. Chemical modifications include cross linking, acetylation, phosphorylation, oxidation, etc. Physical modifications involve pre-gelatinization of starch by drying or heating. Corn or maize starch is the most commonly used starch although tapioca (sago), rice, wheat and potato starches can be employed to modify textures.
39.2.2 Sugars
In the chewing gum manufacture, sugar used shall be of very fine particle size (less than 20 microns) to avoid gritty feel in the mouth. Chewing gum, therefore, has to be made from milled sugar similar to icing sugar. Milled sugar is difficult to handle and if exposed to high humidity they agglomerate. Therefore, the confectioners procure crystalline sugar and mill it on-site and feed the milled sugar directly into the manufacturing process. Sugar is usually added at a rate of about 25% of the gum base. Dextrose monohydrate is sometimes used as an alternative to sucrose in chewing gum. The endothermic heat of solution of dextrose gives a cooling sensation in the mouth, a property that goes well with mint flavours but not with others. To achieve a final TSS content of at least 75%, glucose syrup or doctors are incorporated.
39.2.3 Texturizers
Texturizers are substances that are added to the gum base to modify the mouth feel and facilitate processing. Common texturizers are food grade calcium carbonate or talc. Both of these are less expensive than the other ingredients in gum base. Low cost chewing gum bases contain about 45-55% texturizers whereas high quality chewing gum base contains 18-20%. Bubble gum bases have a texturizer level varying between 30 and 60%. Calcium carbonate is not an acceptable texturizer in products where there is an acid component to the flavour as it reacts with the acid to produce carbon dioxide. Typically acids are only used in fruit-flavoured products, and here, talc must be used as a texturizer.
39.2.4 Humectants
Chewing gum can be spoiled by either loss or gain of moisture. If the gum picks up too much moisture it will become too soft and could darken; if the gum dries out it becomes too hard. Either of these problems can be prevented by wrapping the product in a moisture-proof barrier. However, it is common to add humectants to chewing gum in order to lower the water activity and hence reduce drying out. Common humectants used are sorbitol and glycerol. While sorbitol is a purely vegetable product, glycerol can be produced by the hydrolysis of fat including animal fats, which can cause problems with some religious and ethnic groups. Glycerol can also be produced from petrochemical origin. Excess use of humectants can make the products soft and sticky.
39.2.5 Antioxidants
Gum bases are prone to oxidation and antioxidants help in preventing it. Typically, they contain permitted antioxidants such as butylated hydroxytoulene (BHT), butylated hydroxyanisole (BHA) or tocopherols. However, in India, only BHA maximum up to 250 ppm is permitted in chewing gums as per Food Safety and Standards Rules (2011).
39.2.6 Colours
Permitted colours are invariably added as solutions at the end of the mixing operation in the process. Colours must be acid-fast, resistant to reducing sugars, stable to operating temperatures and should not separate into basic colours during the gel-setting operation.
39.2.7 Flavours
Addition of flavours enhances the acceptability of chewing gums. Adding flavours to warm liquors under atmospheric conditions can lead to loss of volatiles, and hence, in-line mixing is preferable. ‘Medicated’ flavours such as menthol and ‘alcohols’ such as gin are especially susceptible. Small quantities of salt can sometimes be used to enhance the perception of non-fruit flavours. However, the choice of flavouring agent must take into account the stability in the chosen hydrocolloid system.
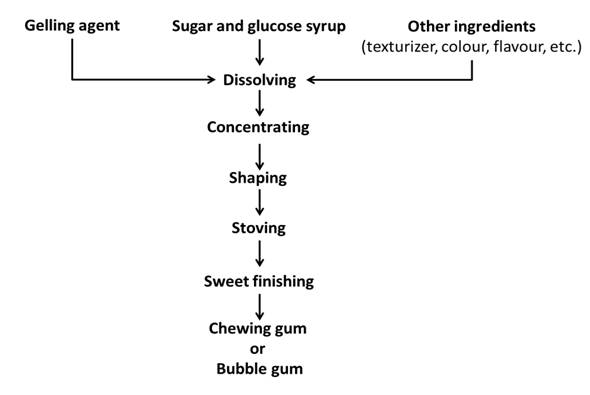
Fig. 39.1 Generalized flow diagram for the manufacture of chewing gum or bubble gum
39.3 Manufacturing of Chewing or Bubble Gum
Gums are prepared by mixing sugar syrup, high dextrose equivalent (DE) glucose syrup, gelling agent or hydrocolloid and other minor but important ingredients such as texturizer, flavours, colours, etc. The generalized manufacturing process for making chewing gum or bubble gum is given in Fig. 39.1. The gelling agent used in the process is cleaned, ground, purified, soaked, made soluble or dissolved or in some way standardized before incorporated in the sugar liquors. In case of bubble gum manufacture, as mentioned earlier, gum base should contain either higher levels of polymers or polymers of high molecular weight that yield a more extensible product able to form bubbles. Whenever hydrocolloids are used in combination, they have to be prepared separately and blended later to avoid coacervation (process of holding organic molecules together by hydrophobic forces from a surrounding liquid). The liquor is prepared from processed sugar syrups. It is necessary to achieve a final liquor of at least 75% TSS to prevent mould growth. As sucrose is saturated at 67% at ambient temperature, glucose syrup, invert sugar or other doctors are used to inhibit crystallization. The main objective in preparing liquor is to produce a high-solids fluid mass that can be shaped. The dissolved ingredients in the syrup are concentrated using plate or film evaporators. The concentrated mass is shaped either by depositing, slabbing or extrusion methods. In depositing method, the product is deposited in starch or starchless moulds of a variety of shapes. Slabbing is an ideal way of producing multi-layered products because each layer can set before the addition of the next. Shaping at the cooled extrusion die has the advantage that all the unit operations are done on the one piece of equipment. Shapes such as laces, round or star strings, sheets, round or star tubes, corrugated ribbons, co-extruded centre-filled strings, etc. can be formed after shaping, the product is dried to a final moisture content, the process which is called as “stoving”. As per FSSR (2011), chewing gum and bubble gum shall not have less than 12.5 and 14.0 percent (by weight) moisture. It is a simple process in which the trays are placed in a heated chamber and hot air is blown over the sheets. The rate at which a given product can be stoved is determined by the size of the sweet, the viscosity of the sweet and the maximum temperature that can be used without damaging the product. Finishing treatments such as oiling and polishing are followed after stoving. Oiling and polishing not only serve to provide extra glossy appearances but also prevent chewing gums from sticking together.
39.4 Common Defects In Chewing Gums
39.4.1 Cloudiness
This is caused by low-grade or undissolved hydrocolloid, coacervation, salting out, calcium precipitation or air entrapment. Use of more water, a longer time or higher temperature for dissolving colloid prevents this defect. Increasing liquor standing time or vacuum deaeration removes entrapped air.
39.4.2 Crystallization
It is normally caused by sucrose crystallization and hence reducing sucrose content or increasing doctoring by adding reducing sugars helps in reducing crystallization.
39.4.3 Mis-shapes
Sweets overbunched while still soft or insufficiently hardened before finishing processes is usually responsible for this defect.
39.4.4 Stickiness
This can be caused by the ERH being too low, excess reducing sugar, variable acidity, incorrect total solids, under cooking or over cooking, insufficient gelatinzation, etc.