Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Lesson 9. Water Treatment
9.1 INTRODCTION
Water is nature's most wonderful, abundant and useful compound. Of the many essential elements for the existence of human beings, animals and plants (wiz. air, water, food, shelter, etc.), water is rated to be of the greatest importance. Without food, human call survives for a number of days, but water is such an essential thing that without it one cannot survive.
Water is not only essential for the lives of animals and plants, but also occupies a unique position in industries. Probably, its most important use as an engineering material is in the 'steam generation '. Water is also used a coolant in power and chemical plants. In addition to it, water is widely used in other fields such as production of steel, rayon, paper, atomic energy, textiles, chemicals, ice, and for air-conditioning, drinking, bathing, sanitary, washing, irrigation, fire-fighting, etc.
9.2 SOURCES OF WATER:
9.2.1 Surface waters:
(i). Rain water is probably the purest form of natural water, since it is obtained as a result of evaporation from the surface water. However, during the journey downwards through the atmosphere, it dissolves a considerable amount of industrial gases (like CO2, SO2, NO2, etc.) and suspended solid particles, both of organic and inorganic origin.
(ii). River water: Rivers are fed by rain and spring waters. Water from these sources flow over the surface of land, dissolves the soluble minerals of the soil and finally falls in rivers. In general, the greater the contact that water has with the soil, or the more soluble the minerals of the soils with which it has come in contact, the greater is the amount of dissolved impurities in river water. River water thus contains dissolved minerals of the soil such as chlorides, sulphates, bicarbonates of sodium, calcium, magnesium and iron. River water also contains the organic matter, derived from the decomposition of plants, and small particles of sand and rock suspension. Thus, river water contains considerable amounts of dissolved as well as suspended impurities.
(iii). Lake water has a more constant chemical composition. It, usually, contains much lesser amount of dissolved minerals than well water, but quantity of organic matter present in it is quite high.
(iv). Sea water is the most impure form of the water. Rivers join sea and throw in the impurities carried by them. Moreover, continuous evaporation of water from the surface of sea makes sea water continuously richer in dissolved impurities. Sea water contains, on an average, about 3.5% of dissolved salts, out of which about 2.6% is sodium chloride. Other salts present are sulphates of sodium; bicarbonates of potassium magnesium and calcium; bromides of potassium and magnesium and a number of other compounds.
Surface water, generally, contains suspended matter, which often contains the disease–producing (or pathogenic) bacteria’s. Hence, such waters as such are not considered to be safe for human consumption.
9.2.2 Underground waters: A part of the rain water, which reaches the surface of the earth, percolates into the earth. As this water journeys downwards, it comes in contact with a number of mineral salts present in the soil and dissolves some of them. Water continues its downwards journey, till it meet a hard rock, when it retreads upwards and it may even come out in the form of ‘spring’.
9.2.3 Spring and well water (or underground water), in general, is clearer in appearance due to the filtering action of the soil, but contain more of dissolved salts. Thus, water from these sources contains more hardness. Usually, underground water is of high organic purity.
9.3 CHARACTERISTICS IMPARTED BY IMPURITIES IN WATER
The natural water is, usually, contaminated by different types of impurities. The characteristic and consequent effects of impurities on the quality of water are discussed under the following three heads:
9.3.1 Physical impurities:
(a) Color in water is caused by metallic substances like salts of iron, manganese, humus materials, tannins, peat, algae, weeds, protozoa, industrial effluents (from paper and pulp, textile, tanneries, etc). Actually, color in water is due to dissolved substances and substances present as fine colloids. The change in color of water is not harmful, unless it is associated with any chemical of toxic nature. Variations in color of water from the same source (say a river) with time often serves as indices of quality of water. Usually, yellowish tinge indicates the presence of chromium and appreciable amount of organic matter. Yellowish-red color indicates the presence of iron; while red-brown color indicates the presence of peaty matter.
(b) Turbidity is due to the colloidal, extremely fine suspension such as clay, slit, finely divided matters (organic and inorganic) micro-organisms like plankton, etc. Turbidity expresses the optical properties of water containing insoluble substances, which scatter light rather than to transmit in straight lines. The turbidity depends not only on the quantity of insoluble substances, but also on their size, shape and refractive index. Turbidity in water can be eliminated by sedimentation, followed by coagulation, filtration, etc.
(c) Taste is, usually, interlinked directly with odor. However, in some waste water, taste is not accompanied by odor. Thus, presence of dissolved mineral in water produces taste, but not odor. For example;
(i) Bitter taste can be due to the presence of iron, aluminum, manganese, sulphate or excess of lime.
(ii) Soapy taste can be due to the presence of large amount of sodium bicarbonate.
(iii) Brackish taste is due to the presence of unusual amount of salts.
(iv) Palatable taste is due to the presence of dissolved gases (CO2) and minerals (like nitrates) in water.
(d) Odor in water is undesirable for domestic as well as industrial purposes. Disagreeable odor in water may be caused by the presence of living organisms, decaying vegetation including algae, bacteria, fungi and weeds. The receiving water may be offensive where heavy pollution is caused by sewage/industrial effluents. The most common disagreeable odor in water bodies is due to presence of small quantity of sulphides. The causes of odor in polluted rivers are;
(i) Presence of inorganic and organic compounds of N, S and P and the putrefaction of proteins and other organic materials present in sewage;
(ii) Industrial effluents containing organic substances such as alcohols, aldehydes, phenols, esters, ketones, etc. flowing into the water bodies.
Besides these : (i) presence of algae in water bodies impart a strong grassy odor, due to the liberation of traces of essential oils from their bodies, (ii) growth of iron and sulphur bacteria may produce offensive odors, (iii) presence of colloidal vegetable matter in surface-water evolves a faint flavor, called 'peaty' odor, (iv) clay and sand in finely divided state impart a faint earthy odor, (v) improper bituminous coating on cast iron main pipes may give off a tarry odor to water which passes through it, (vi) a faint odor is imparted to water passing through new iron or galvanized. This odor is due to the impure hydrogen.
9.3.2 Chemical impurities in water includes: (1) inorganic and organic chemicals (some are toxic immature) released from dyes, paints, and vanishes, drugs, insecticides, pesticides, detergent, pulp and textiles, industries, canneries, etc. All these pollute water bodies, (2) acids discharged in water by DDT, high explosives, battery, industries, etc. The use of this type of contaminated water causes harmful effects on health of human-beings.
(a) Acidity is not any specific pollutant and it simply determines the power to neutralize hydroxyl ions and is, usually, expressed in terms of ppm (or mg/L) of calcium carbonate equivalent. Surface waters and ground waters attain acidity from industrial wastes like acid, mine, drainage, pickling liquors, etc. Usually, acidity is caused by the presence of free CO2, mineral acids (e.g., H2SO4) and weakly dissociated acids. Mineral acids are released when iron and aluminum salts hydrolyze.
(b) Gases: (i) All natural waters contain dissolved atmosphere CO2. Its solubility depends upon temperature, pressure and dissolved mineral content of water. Also polluted waters acquire CO2 from the biological oxidation of organic matter. It’s presence in water is of no significance.
(ii) Concentration of dissolved atmospheric O2 in waters depends on temperature, pressure and salt content in water. Dissolved O2 in industrial waters is nuisance, since it induces corrosion reactions. On the other hand, dissolved O2 in water is essential to the life of aquatic organisms such as fishes.
(iii) Dissolved NH3 in water arises from the decomposition (aerobic or anaerobic) of nitrogenous organic matter. Polluted waters and sewages contain nitrogen in the form of nitrogenous organic compounds and urea, which are partially converted into NH3.
(c) Mineral matters have origin from rocks and industrial effluents. These include mineral acids, Ca2+, Mg2+, Na+, K+, Fe2+, CO32-, Mn2+, HC03 - , Cl-, SO42-, N03- , F-, SiO2 etc. However, from industrial point of view, alkalinity and hardness are important.
(3) Biological impurities are algae, pathogenic bacteria, fungi, viruses, pathogens, parasite worms, etc. The source of this contamination is discharge of domestic and sewage wastes, excreta (from man, animals and birds) etc.
(a) Micro-organisms are, usually; abundant in surface waters, but their count is often quite low or even nil in deep-well waters. The commonest type of micro-organisms from the point of treatment are algae, fungi and bacteria, which often form 'slime ', thereby causing fouling as well as corrosion. The slime so-formed clogs the spray nozzles and screens of the circulating pumps in air-conditioning and other industrial plants. The growth of micro-organisms takes place at temperature between 20-35oC. In order to control the micro-organisms, chemical treatment like chlorination is done.
(b) Water bodies in water includes: (i) bacteria, organisms inhabitation the bottom sludge, and (ii) organisms and planktons developed at the water surface. These are inhabitated by different group of worms like flat worms, hair worms tiny round worms, rotifers, etc. Usually, most worms are parasite in nature and these water bodies are not only harmful to fishes, but also to human health.
9.4 HARDNESS OF WATER
Hardness in water is that characteristic, which prevents the lathering of soap. This is due to presence in water of certain salts of calcium, magnesium and other heavy metals dissolved in it. A sample of hard water, when treated with soap (sodium or potassium salt of higher fatty acid like oleic, palmitic or stearic) does not produce lather, but on the other hand forms a white scum or precipiate. This precipitate is formed, due to the formation of insoluble soaps of calcium and magnesium. Typical reactions of soap (sodium stearate) with calcium chloride and magnesium sulphate are depicted as follows:
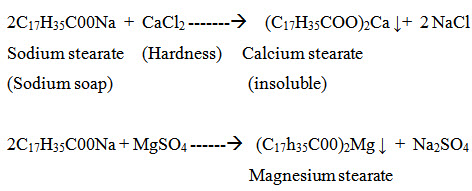
Thus, water which does not produce lather with soap solution readily, but forms a white curd, is called hard water. On the other hand, water which lathers easily on shaking with soap solution, is called soft water. Such water, consequently, does not contain dissolved calcium and magnesium salts in it.
(1) Temporary or carbonate hardness is caused by the presence of dissolved bicarbonates of calcium, magnesium and other heavy metals and the carbonate of iron. Temporary hardness is mostly destroyed by mere boiling of water, when bicarbonates are decomposed, yielding insoluble carbonates or hydroxides, which are deposited as a crust at the bottom of vessel. Thus;
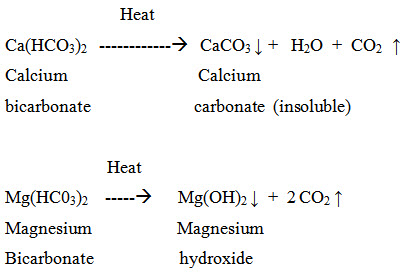
(2) Permanent or non-carbonate hardness is due to the presence of chlorides and sulphates of calcium, magnesium, iron and other heavy metals. Unlike temporary hardness, permanent hardness is not destroyed on boiling.
9.5 EQUIVALENTS OF CALCIUM CARBONATE
The concentration of hardness as well as non-hardness constituting ions are, usually expressed in terms of equivalent amount of CaCO3, since this mode pemlits the multiplication and division of concentration, when required. The choice of CaCO3 in particular is due to its molecular weight is 100 (equivalent weight = 50) and moreover, it is the most insoluble salt that can be precipitated during water treatment.
The equivalents of CaC03,
[Mass of hardness producing-substance] x [Chemical equivalent of CaCO3]
=- --------------------------------------------------------------------------------------------------------------------------------------------
Chemical equivalent of hardness-producing substance
= Mass of hardness-producing substance x 50
-----------------------------------------------------------------
Chemical equivalent of hardness-producing substance
9.6 UNITS OF HARDNESS
(1) Parts per million (ppm) is the parts of calcium carbonate equivalent per 106 parts of water, i.e., 1 ppm = 1 part of CaCO3 eq. hardness in 106 parts of water.
(2) Milligrams per liter (mg/L) are the number of milligrams of CaCO3 equivalent hardness present per liter of water. Thus;
1 mg/L = 1 mg of CaCO3 eq. hardness of 1 L of water
But 1 L of water weighs
= 1 kg = 1,000 g = 1,000 x 1,000 mg = 106 mg.
. . . 1mg/L = 1 mg of CaCO3 eq. per 106 mg of water.
= 1 part of CaCO3 eq. per 106 parts of water = 1 ppm
(3) Clarke's degree (oCI) is number of grains (l/7000 1b) of CaCO3 equivalent hardness per gallon(10lb) of water. Or it is parts of CaCO3 equivalent hardness per 70,000 parts of water. Thus:
lo Clarke = 1 grain of CaC03 eq. hardness per gallon of water.
1o CI= 1 part of CaC03 eq. hardness per 70,000 parts of water.
(4) Degree French (oFr) is the parts of CaCO3 equivalent hardness per 105 parts of water. Thus:
1o Fr = 1 part of CaCO3 hardness eq. per 105 parts of water.
(5) Mille equivalent per liter (meq/L) is the number of mill equivalents of hardness present per liter. Thus;
1 meq/L = 1 meq of CaCO3 per L of water
= 10-3 x 50 g of CaCO3 eq. per liter
= 50 mg of CaCO3 eq. per liter
= 50 mg/L of CaCO3 eq. = 50 ppm.
Relationship between various units of hardness:
1 ppm = 1 mg/L = 0.1o Fr = 0.07'oCl = 0.02 meq/L
1 mg/L =1 ppm = 0.1o Fr = 0.1o Fr = 0.02 meq/L
1o Cl = 1.433o Fr = 14.3 ppm = 14.3 mg/L = 0.286 meq/L
1o Fr = 10 ppm =10 mg/L =0.07o Cl = 0.2 meq/L
1meq/L = 50 mg/L =50ppm = 5o Fr = 0.35o Cl