Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Lesson 18 Enzymes-I
Introduction
In this lesson, we will study about enzymes, their classification and characteristics.
18.2 Enzymes
Enzymes are biologic polymers which catalyze biochemical reactions. They are functional units of cell metabolism. Most of the enzymes are proteins. Measurement of activities of certain enzymes is an important tool in diagnosing diseases. Enzymes are important practical tools in medicine, chemical industry, food processing and agriculture.
18.3 Constituents of Enzymes
Almost all of the enzymes are proteins. Some enzymes (e.g. pancreatic ribonuclease) are made up of only polypeptides and contain no groups or atoms other than amino acid residues. Some enzymes require additional chemical component called cofactor. The cofactor can be of two types:-
- Inorganic ions like Fe2+,Zn2+,Mg2+ etc.
- Coenzyme: which is a complex organic group.
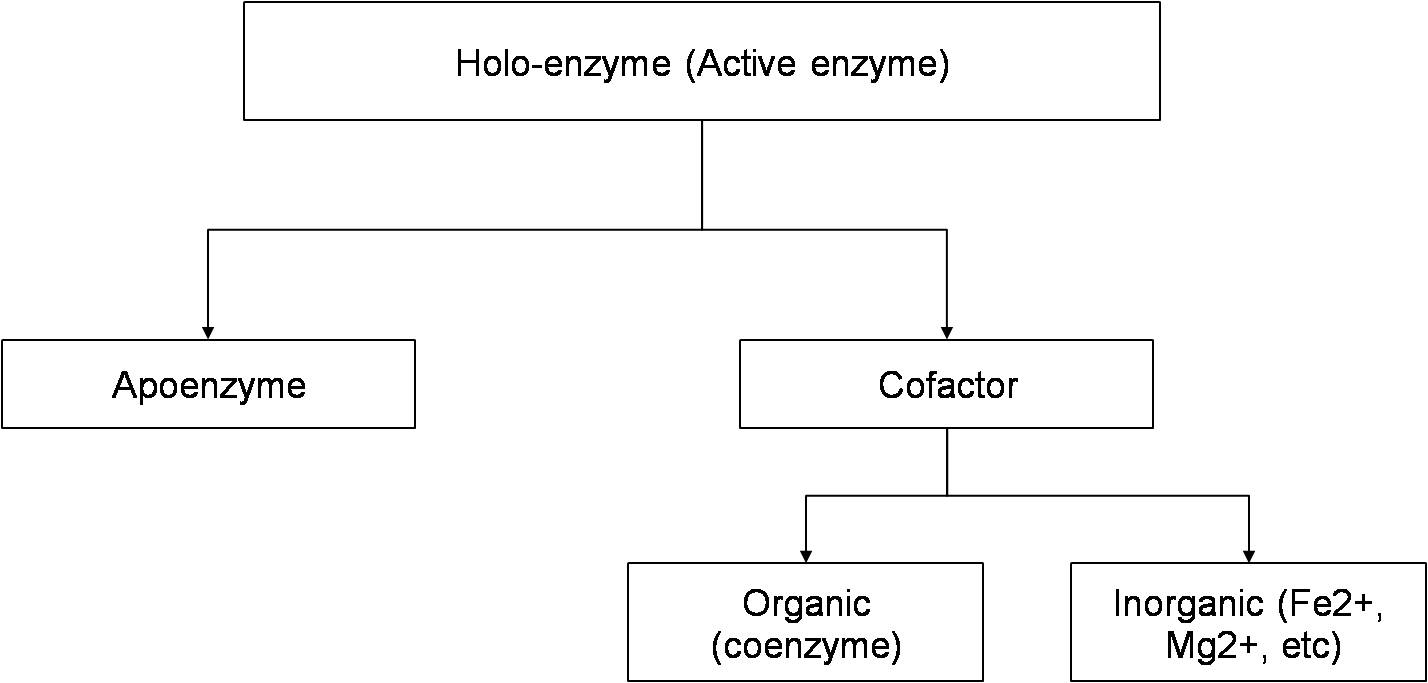
Figure 18.1 Figure showing constituents of enzyme molecules
There are some enzymes which require both coenzyme and metal ions for activity. In some enzymes, coenzymes or metal ions are loosely bound to the protein. In other enzymes it is tightly and permanently bound and is called prosthetic group. A complete catalytically active enzyme together which its coenzyme or metal ion is called holoenzyme. The protein part of an enzyme is known as apoenzyme. Coenzyme and metal ions are stable to heat while the protein part is unstable and denatured on heating.
In an enzyme, the inorganic part is essential for activity of some enzymes, while the coenzyme functions as carries of specific functional groups.
Table18.1 Some enzymes containing Inorganic elements as cofactors
|
Inorganic Part |
Enzyme |
|
Zn2+ |
Alcohol dehydrogenase |
|
Ni 2+ |
Urease |
|
Mg2+ |
Hexokinase |
|
K+, Mg2+ |
Pyruvate kinase |
Table18.2 Coenzymes which function as carries of functional group
|
Coenzymes |
Group of atoms transferred |
|
Thiamine pyrophosphate |
Aldehydes |
|
Coenzyme A |
Acyl groups |
|
Pyridoxal Phosphate |
Amino groups |
|
Co enzyme B12 |
H atoms and alkyl groups |
18.4 Classification of Enzymes
Enzymes are classified into six major groups depending on the type of reactions they catalyze. The complete rules of nomenclature and classification can be found here.
Table18.3 International classification of enzymes
|
No. |
Class |
Type of reaction Catalyzed |
Examples |
|
1. |
Oxidoreductases |
Transfer of electrons |
Alcohol dehydrogenase, peroxidases, catalases, etc |
|
2. |
Transferases |
Transfer of groups.(e.g. methyl, NH2) |
Phosphorylases, transaminases, hexokinase, etc. |
|
3 |
Hydrolases |
Hydrolytic cleavage of C-C, C-N,C-O & some other bonds |
α & β amylase, urease, etc |
|
4. |
Lysases |
Addition of groups to double bond or cleavage of C-C, C-N, C-O & other bonds by elimination, resulting in formation of double bonds or rings. |
Carboxylysases |
|
5. |
Isomarases |
Geometric or structural changes within one molecules |
Racemases, cis-trans isomerases, etc |
|
6. |
Ligases |
Joining together of two molecules coupled with hydrolysis of diphosphate bond in ATP or similar molecules |
Pyruvate carboxylase |
18.5 Characteristics of Enzymes
18.5.1 Enzymes enhance rate of chemical reaction by lowering their activation energy.
Explanation:- During a chemical reaction the reactant molecules are first activated(their energy is increased). The difference in energy between activated state and initial state is known as activation energy. Once the molecules are activated they are converted to products. Enzymes lower the activation energy by providing an alternative path to reach that state. Thus in given time period more number of molecules reach the activation state and reaction is catalyzed.
18.5.2 The substrate concentration has significant effect on rate of enzyme catalyzed reactions and a quantitative relationship exist between the two.
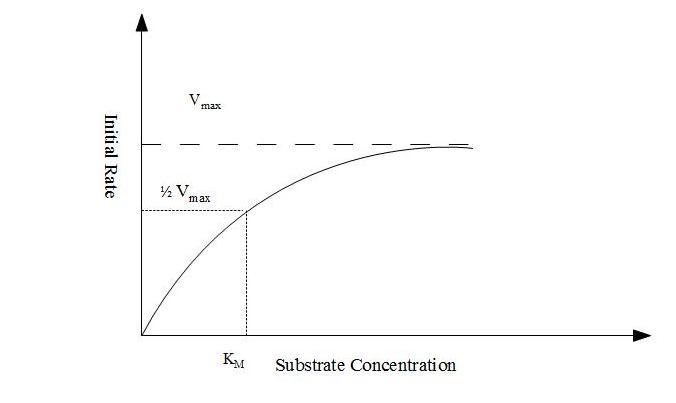 Figure 18.2 Figure showing effect of substrate concentration on initial rate of reaction
Figure 18.2 Figure showing effect of substrate concentration on initial rate of reaction
Explanation:- Consider a case where enzyme concentration is held constant. Also for simplicity consider that the enzyme acts on only on the substrate. If substrate concentration is low, the initial rate of reaction will also be low but it will increase as the former increases. As the substrate concentration increases, the increase in rate becomes smaller and smaller and approaches a constant value. At this rate the enzyme is saturated with the substrate. This phenomenon is known as the saturation effect exhibited by enzyme molecules.
If an enzyme acts on only one substrate, the relationship between initial rate of reaction and substrate concentration (known as Micheles-Menten equation) can be written as
Where,
Vo = Initial rate at substrate concentration [S]
Vmax = Maximum rate
KM = Micheles-Menten constant
18.5.3 Enzymes have an optimum pH at which their activity is maximum.
For example, pepsin is an enzyme which is released in stomach and hydrolyses peptide bonds of proteins has its maximum activity at pH 1.5. The enzyme catalse has optimum pH 7.6.
18.5.4 The activity of enzyme can be determined quantitatively.
Explanation:- There are various methods of determining enzyme activity. By international agreements, 1.0 unit of enzyme activity is defined as that amount causing transformation of 1.0 micromole of a substrate per minute at 25°C under optimum conditions.
18.5.5 Enzymes are specific in their action.
Explanation:- some enzymes act on a a single substance and will not attack other closely related molecules. There are some enzymes which are broad in their action and will act on compounds having common structural feature.
18.5.6 Most enzymes can be inhibited by specific chemical reagents
References & Further Reading:
1. Lehninger A. (1987), “Principles of Biochemistry”. B S Publishers & Distributers, pp. 207-221.
2. http://www.chem.qmul.ac.uk/aubmb/enzymes/rules.html
3. Satyanarayana U. & Chakrapani U. (2011), “Biochemistry”. Books and Allied (P) Ltd. pp. 85-96.