Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Lesson 23 Proteins
23.1 Introduction
The name protein is derived from Greek word “proteios” which means first or prime or principal. They are so named because proteins are the main constituents of all living cells. All protein molecules have Nitrogen alongwith Carbon, Hydrogen and Oxygen in their molecules (unlike carbohydrates and lipids which are primarily made up of Carbon, Hydrogen and Oxygen). Some protein molecules contain small amounts of Sulphur, Phosphorous and minerals. Chemically proteins are polymers. They are polyamides of α-amino acids.
23.2 Biological Importance
Some proteins act as enzymes and hormones. Many important processes like growth, digestion, metabolism, excretion etc. are controlled by proteins. Proteins help in maintaining osmotic pressure and pH of body fluids. Some proteins act as natural defence agents. 1 gm of protein yields 4 kcal of energy. Proteins should provide 10-14% of total energy of the diet.
23.3 Amino Acids
Amino acids are the building blocks of protein molecules. There are 20 amino acids which are commonly found in proteins. A single protein molecule contains hundreds or thousands of amino acids. Thus, the number of protein molecules which can be formed from different permutations and combinations of the 20 amino acids is infinite.
Amino acids contain two functional groups, basic amino (-NH2) group and acidic (-COOH) group. The third group is known as the side chain and designated as (-R). The general structure of amino acid molecule is as follows:
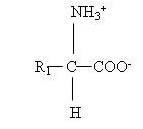
23.3.1 Classification of Amino acids
The amino group and carboxylic group are common to all acids, hence the amino acids can be classified on the basis of their R groups as shown in Table :
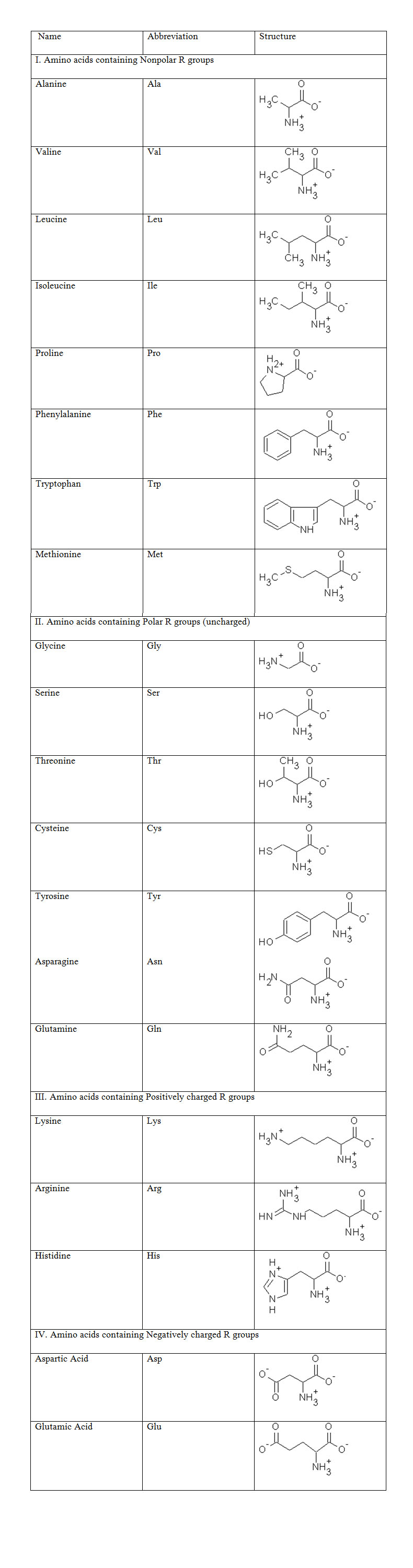
All amino acids except glycine have a chiral Carbon atom (all four groups attached to this C atom are different) and can have D- and L- configurations. All the naturally occuring amino acids have L- configuration. All amino acids are ionized at neutral pH. Amino acids exhibit reactions of both amino and carboxylic acid groups.
23.4 Peptides
α-amino group of one amino acid reacts with carboxyl group of another, with loss of a water molecule to form a peptide bond between the two.
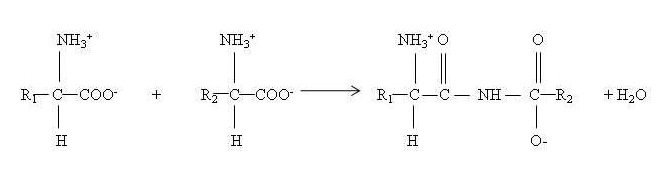
When 2-10 amino acids are joined together, resulting molecule is called as oligopeptide, with more than 10 amino acids it is called polypeptide and when the number of amino acids is more than about 100, it is called a protein.
23.5 Proteins
Proteins can be classified according to their composition into simpple proteins and conjugate proteins. After hydrolysis simple proteins yield only amino acids while conjugated proteins yield amino acids and inorganic or organic groups known as prosthetic groups. The simple proteins are further classified into Albumins, Globumins, Glutelins, Prolamines, Histones, Protamines, Sclero-proteins (albuminoids), etc.
23.5.1 Structure of Protein
The structure of protein molecules is considered at four levels – primary, secondary, tertiary and quaternary
23.5.1.1 Primary Structure
It is the way in which amino acids are sequentially attached in the polypeptide chains by covalent bonds. The sequence of amio acids in a protein molecule is unique and is responsible for many of its properties e.g., replacement of a single amino acid in a protein chain can render it inactive. The secondary and tertiary structures of a protein molecule depend to a large extent on primary structure. The primary structure can be foud out by repeated hydrolysis of protein molecule and analyzing the fragments obtained.
23.5.1.2 Secondary Structure
A peptide chain is not stable and folds itself under the influence of 3-dimensional nature of the bonds. The 3-dimensional manner in which relatively close members of the protein chain are arranged is referred to as secondary structure. The secondary structure of the protein depends upon structural characteristics of peptide bonds. The secondary structure can be found out by x-ray diffraction technique. The various types of secondary structures are α-helix, collagen helix, β-pleated sheets, etc.
23.5.1.3 Tertiary Structure
It defines specific 3-dimensional configuration of the protein molecule. This involves folding of repeating units of secondary structure. The folded portions are held together by hydrogen bonds, electrostatic attraction between oppositely charged groups, hydrophobic interactions and covalent disulphide linkages. In tertiary structure, all polar groups are at surface of the molecule and non-polar groups in the interior, which is the reason for their solubility in aqueous solutions.
23.5.1.4 Quaternary Structure
When a protein molecule contains only a single polypeptide chain, it has only three levels of structure (primary, secondary and tertiary). When a protein molecule has two or more polypeptide chains, the structure formed when individual polypeptide chains interact to form the protein molecule is known as quaternary structure. The bonding mechanism involved in holding the different polypeptide chains together are hydrogen bonds, electrostatic attraction and hydrophobic interactions.
23.5.2 Properties of Proteins
Protein molecules behave as electrolytes. Their solubility is affected by pH, ionic strength, temperature and dielectric properties of solvent. This property is used in isolation and purification of protein molecules.
Proteins can be hydrolyzed by acids, alkalies or enzymes. Acid or alkaline hydrolysis causes racemization of amino acids and loss of certain amino acids. Enzyme hydrolysis prevents destruction of amino acids.
Proteins are putrefied by microorganisms during which microbial proteases act on protein molecules resulting in destruction of amino acids leading to production of offensive odous and toxins, and changes in flavour and texture.
Proteins can be denatured. Denaturation is a process in which quaternary, tertiary or secondary structures are modified in such a way that the properties responsible for identity of the protein molecule are lost. Denaturation does not affect primary structure. Denaturation is reversible if mild agents are used. Some of the agents which cause denaturation are heat, stirring, shaking, high pressure, ultraviolet radiation, pH changes, detergents, etc.
Dry proteins have the ability to absorb water and can form a gel like structure.
References & Further Reading:
1. Satyanarayana U. & Chakrapani U. (2011), “Biochemistry”. Books and Allied (P) Ltd. pp. 43-67.
2. N. Shakuntala Manay & M. Shadaksharaswamy (2001), “Foods: Facts and Principles”. New Age International Publishers, pp. 43-58.