Site pages
Current course
Participants
General
MODULE 1. Magnetism
MODULE 2. Particle Physics
MODULE 3. Modern Physics
MODULE 4. Semicoductor Physics
MODULE 5. Superconductivty
MODULE 6. Optics
5 April - 11 April
12 April - 18 April
19 April - 25 April
26 April - 2 May
LESSON 13. Paschan Back Effect & Introduction to Raman Spectroscopy
Paschan Back Effect
In the presence of an external magnetic field, the energy levels of atoms are split. This splitting is described well by the Zeeman effect if the splitting is small compared to the energy difference between the unperturbed levels, i.e., for sufficiently weak magnetic fields. This can be visualized with the help of a vector model of total angular momentum. If the magnetic field is large enough, it disrupts the coupling between the orbital and spin angular momentum, resulting in a different pattern of splitting. This effect is called the Paschen-Back effect
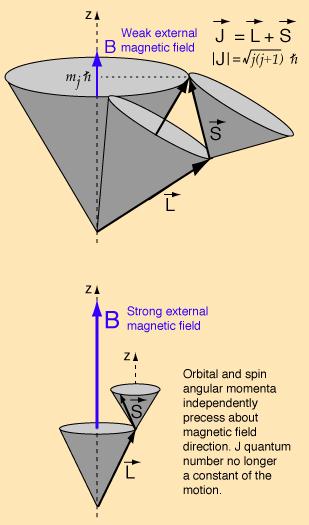
In the weak field case the vector model at left implies that the coupling of the orbital angular momentum L to the spin angular momentum S is stronger than their coupling to the external field. In this case where spin-orbit coupling is dominant, they can be visualized as combining to form a total angular momentum J which then precesses about the magnetic field direction
In the strong-field case, S and L couple more strongly to the external magnetic field than to each other, and can be visualized as independently precessing about the external field direction.
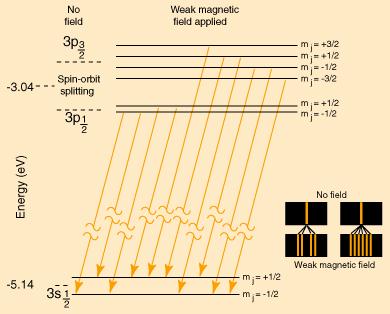
For reference, the sodium Zeeman Effect is reproduced below to show the nature of the magnetic interaction for weak external magnetic fields.
The following is a model of the changes in the pattern if the magnetic field were strong enough to decouple L and S. The resulting spectrum would be a triplet with the center line twice the intensity of the outer lines.
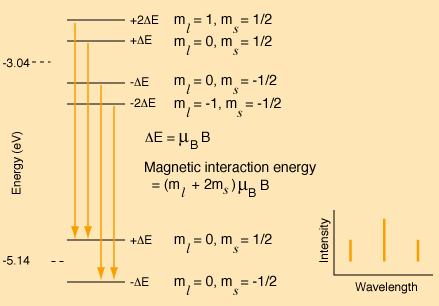
To create this pattern, the projections of L and S in the z-direction have been treated independently and the ms multiplied by the spin g-factor. The energy shift is expressed as a multiple of the Bohr magnetrons mB. The selection rules explain why the transitions shown are allowed and others not.
Sodium was used as the basis of the model for convenience, but the fields required to create Paschen-Back conditions for sodium are unrealistically high. Lithium, on the other hand, has a spin-orbit splitting of only 0.00004 eV compared to 0.0021 eV for sodium. Such small energy values are sometimes expressed in "wave numbers", or 1/l in cm-1. In these units the lithium separation is about 0.3 cm-1and the sodium separation is about 17 cm-1 .The Paschen-Back conditions are met in some lithium spectra observed on the Sun, so this effect does have astronomical significance.
Raman Spectroscopy
Introduction
Raman spectroscopy is a spectroscopic technique based on inelastic scattering of monochromatic light, usually from a laser source. Inelastic scattering means that the frequency of photons in monochromatic light changes upon interaction with a sample. Photons of the laser light are absorbed by the sample and then reemitted. Frequency of the reemitted photons is shifted up or down in comparison with original monochromatic frequency, which is called the Raman Effect. This shift provides information about vibrational, rotational and other low frequency transitions in molecules. Raman spectroscopy can be used to study solid, liquid and gaseous samples.
Origins of Raman
The Raman Effect is based on molecular deformations in electric field E which is determined by molecular polarizability α. The laser beam can be considered as an oscillating electromagnetic wave with electrical vector E. Upon interaction with the sample it induces electric dipole moment P = αE which deforms molecules. Because of periodical deformation, molecules start vibrating with characteristic frequency υm.
Amplitude of vibration is called a nuclear displacement.
In other words, monochromatic laser light with frequency υ0 excites molecules and transforms them into oscillating dipoles. Such oscillating dipoles emit light of three different frequencies
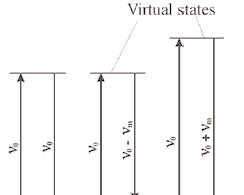
when:
-
A molecule with no Raman-active modes absorbs a photon with the frequency υ0. The excited molecule returns back to the same basic vibrational state and emits light with the same frequency υ0 as an excitation source. This type if interaction is called an elastic Rayleigh scattering.
-
A photon with frequency υ0 is absorbed by Raman-active molecule which at the time of interaction is in the basic vibrational state. Part of the photon’s energy is transferred to the Raman-active mode with frequency υm and the resulting frequency of scattered light is reduced to υ0 - υm. This Raman frequency is called Stokes frequency, or just “Stokes”.
-
A photon with frequency υ0 is absorbed by a Raman-active molecule, which, at the time of interaction, is already in the excited vibrational state. Excessive energy of excited Raman active mode is released, molecule returns to the basic vibrational state and the resulting frequency of scattered light goes up to υ0 + υm. This Raman frequency is called Anti- Stokes frequency, or just “Anti-Stokes”.
About 99.999% of all incident photons in spontaneous Raman undergo elastic Rayleigh scattering. This type of signal is useless for practical purposes of molecular characterization. Only about 0.001% of the incident light produces inelastic. Raman signal with frequencies υ0 ± υm. Spontaneous Raman scattering is very weak and special measures should be taken to distinguish it from the predominant Rayleigh scattering. Instruments such as notch filters, tunable filters, laser stop apertures, double and triple spectrometric systems are used to reduce Rayleigh scattering and obtain high-quality Raman spectra.