Site pages
Current course
Participants
General
MODULE 1. Magnetism
MODULE 2. Particle Physics
MODULE 3. Modern Physics
MODULE 4. Semicoductor Physics
MODULE 5. Superconductivty
MODULE 6. Optics
5 April - 11 April
12 April - 18 April
19 April - 25 April
26 April - 2 May
LESSON 24. High Temperature SuperConductor (HTS)
Introduction to High \[{T_c}\] superconductors
Basic concept of High Temperature Superconductors (HTS)
-
High-temperature superconductors are materials that behave as superconductors at unusually high temperatures.
-
Symbolically, it is denoted by high- \[{T_c}\] or HTS.
-
The first high- \[{T_c}\] superconductor was discovered in 1986 by the researchers Karl Müller and Johannes Bednorz.
-
The “ordinary” or “metallic” superconductors usually have transition temperatures (temperatures below which they exhibit the superconductivity phenomena) below 30 K (-243.2°C ).
-
The HTS have been observed with transition temperatures as high as 138 K (-135°C ).
-
The compounds of Copper and Oxygen (so-called "cuprates") were believed to have HTS properties, and the term high-temperature superconductor was used interchangeably with cuprites superconductor for compounds such as Bismuth Strontium Calcium Copper Oxide (BSCCO) and Yttrium Barium Copper Oxide (YBCO).
-
However, several iron-based compounds are now known to be superconducting at high temperatures.
History
The phenomenon of superconductivity was discovered by Kamerlingh Onnes in 1911, in metallic Mercury below 4 K (-269.15°C ). For seventy-five years after that, researchers attempted to observe superconductivity at higher and higher temperatures.
In 970, superconductivity was observed in certain metal oxides at temperatures as high as 13 K (-260.2°C ), which were much higher than those for elemental metals.
In 1987, K Alex Müller and J. Georg Bednorz were exploring a new class of ceramics for superconductivity. Bednorz encountered a Barium-doped compound of Lanthanum and Copper oxide whose resistance dropped down to zero at a temperature around 35 K (-238.2°C).
The superconductor with the highest transition temperature that has been confirmed by more than one independent research groups is mercury barium calcium copper oxide ( \[HgB{a_2}C{a_2}C{u_3}{O_8}\] ) at around 133 K.
Crystal structures of high-temperature ceramic superconductors
- The structure of high- \[{T_c}\] Copper oxide or cuprate superconductors are often closely related to perovskite structure [A perovskite structure is any material with the same type of crystal structure as Calcium Titanium Oxide ( \[CaTi{O_3}\] )].
- One of the properties of the crystal structure of oxide superconductors is an alternating multi-layer of \[Cu{O_2}\] planes with superconductivity taking place between these layers.
- This structure causes a large anisotropy in normal conducting and superconducting properties, since electrical currents are carried by holes induced in the oxygen sites of the \[Cu{O_2}\] sheets.
\[YBaCuO\] Superconductors Fig 15(1)
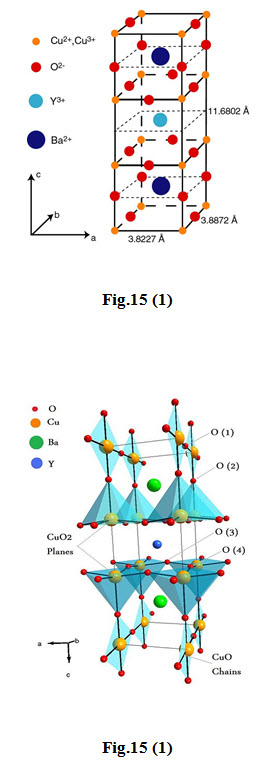
The first superconductor found with \[{T_c}\] > 77 K (liquid nitrogen boiling point) is Yttrium Barium Copper Oxide ( \[YB{a_2}C{u_3}{O_{7 - x}}\] )
The proportions of the 3 different metals in the \[YB{a_2}C{u_3}{O_{7}}\] superconductor are in the mole ratio of 1:2:3 for yttrium to barium to copper, respectively. So it is referred to as the 123 superconductor.
The unit cell of \[YB{a_2}C{u_3}{O_{7}}\] consists of three pseudocubic elementary perovskite unit cells.
Each perovskite unit cell contains a Y or Ba atom at the center: Ba in the bottom unit cell, Y in the middle one, and Ba in the top unit cell.
Thus, Y and Ba a are stacked in the sequence [Ba - Y - Ba ] along the c-axis.
All corner sites of the unit cell are occupied by Cu, which has two different coordination, Cu(1) and Cu(2) , with respect to oxygen.
There are four possible crystallographic sites for oxygen: O(1) , O(2) , O(3) and O(4) . The coordination polyhedral of Y and Ba with respect to oxygen is different.
The tripling of the perovskite unit cell leads to nine oxygen atoms, whereas \[YB{a_2}C{u_3}{O_7}\] has seven oxygen atoms and, therefore, is referred to as an oxygen-deficient perovskite structure. The structure has a stacking of different layers: \[\left( {CuO} \right)\left( {BaO} \right)\left( {Cu{O_2}} \right)\left( Y \right)\left( {Cu{O_2}} \right)\left( {BaO} \right)\left( {CuO} \right)\] .
One of the key features of the unit cell of \[YB{a_2}C{u_3}{O_{7 - x}}\] is the presence of two layers of \[Cu{O_2}\] . The role of the Y plane is to serve as a space between two \[Cu{O_2}\] planes.
In \[YBCO\] , the \[Cu\] - \[O\] chains are known to play an important role for superconductivity.
\[{T_c}\] is maximal near 92 K when \[x\] \[ \approx\] 0.15 and the structure is orthorhombic.
Superconductivity disappears at \[x\] \[ \approx\] 0.6, where the structural transformation of \[YBCO\] occurs from orthorhombic to tetragonal.
\[{B_i}\] - , \[Tl\] - and \[{H_g}\] - based high- \[{T_c}\] superconductors
-
The crystal structure of \[{B_i}\] - , \[Tl\] - and \[{H_g}\] - based high- \[{T_c}\] superconductors are very similar. Like \[YBCO\] ,
-
It is perovskite-type feature and the presence of \[Cu{O_2}\] layers also exists in these superconductors.
-
However, unlike \[YBCO\] , - \[Cu\] - \[O\] chains are not present in these superconductors. The superconductor has an orthorhombic structure, whereas the other high- superconductors have a tetragonal structure.
The \[B{i_2}\]-\[Sr\] -\[Ca\] -\[Cu\] -\[O\] -system has three superconducting phases forming a homologous series as \[B{i_2}S{r_2}C{a_{n - 1}}C{u_n}{O_{4 + 2n + x}}\] (n = 1, 2 and 3)
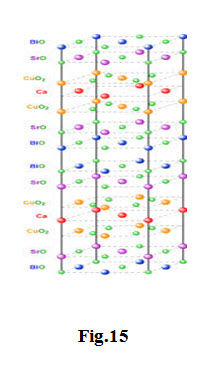
These three phases are \[Bi\]-2201, \[Bi\]-2212 and \[Bi\]-2223, having transition temperatures of 20, 85 and 110 K, respectively, where the numbering system represent number of atoms for \[Bi\], \[Si\] , \[Cu\] Ca and Cu respectively.
The two phases have a tetragonal structure which consists of two sheared crystallographic unit cells. The unit cell of these phases has double \[Bi - O\] planes which are stacked in a way that the Bi atom of one plane sits below the oxygen atom of the next consecutive plane. The Ca atom forms a layer within the interior of the \[Cu{O_2}\] layers in both \[Bi\]-2212 and \[Bi\]-2223; there is no \[Ca\] layer in the \[Bi\]-2201 phase. The three phases differ with each other in the number of \[Cu{O_2}\] planes; \[Bi\]-2201, \[Bi\]-2212 and \[Bi\]-2223 phases have one, two and three \[Cu{O_2}\] planes, respectively. The c axis of these phases increases with the number of \[Cu{O_2}\] planes (see table below). The coordination of the \[Cu\] atom is different in the three phases. The \[Cu\] atom forms an octahedral coordination with respect to oxygen atoms in the 2201 phase, whereas in 2212, the \[Cu\] atom is surrounded by five oxygen atoms in a pyramidal arrangement. In the 2223 structure, \[Cu\] have two co-ordinations with respect to oxygen: one \[Cu\] tom is bonded with four oxygen atoms in square planar configuration and another \[Cu\] atom is coordinated with five oxygen atoms in a pyramidal arrangement.
\[Tl - Ba - Ca - Cu - O\] Superconductor:
-
The first series of the \[TL\] -based superconductor containing one \[TL\]–\[O\] layer has the general formula \[TlB{a_2}C{a_{n - 1}}C{u_n}{O_{2n + 3}}\] whereas the second series containing two \[TL\] –\[O\] layers has a formula of \[T{l_2}B{a_2}C{a_{n - 1}}C{u_n}{O_{2n + 4}}\] with n = 1, 2 and 3
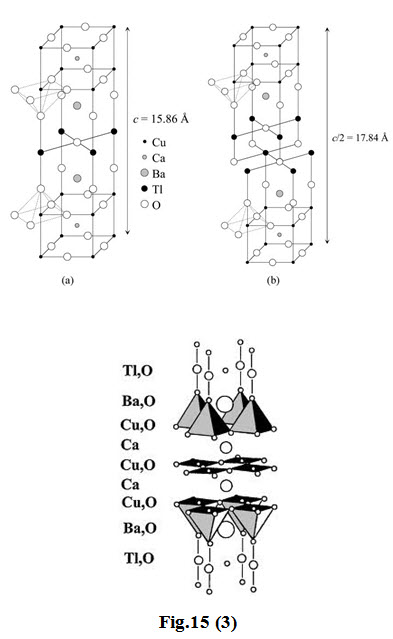
-
In the structure of \[T{l_2}B{a_2}Cu{O_6}\] ( \[TL\]-2201), there is one \[Cu{O_2}\] layer with the stacking sequence ( \[TL\]–\[O\] ) ( \[TL\]–\[O\] ) ( \[Ba\]–\[O\] ) ( \[Cu\]–\[O\] ) ( \[Ba\]–\[O\] ) ( \[TL\]–\[O\] )( \[TL\]–\[O\] ).
-
In \[T{l_2}B{a_2}CaC{u_2}{O_8}\] ( \[TL\]-2212), there are two \[Cu\]–\[O\] layers with a \[Ca\] layer in between. Similar to the \[T{l_2}B{a_2}Cu{O_6}\] structure, \[TL\]–\[O\] layers are present outside the \[Ba\]–\[O\] layers.
-
In \[T{l_2}B{a_2}C{a_2}C{u_3}{O_{10}}\] ( \[TL\]-2223), there are three \[Cu{O_2}\] layers enclosing \[Ca\] layers between each of these.
-
In \[TL\] -based superconductors, \[{T_c}\] is found to increase with the increase in \[Cu{O_2}\] layers. However, the value of \[{T_c}\] decreases after four layers in \[TlB{a_2}C{a_{n - 1}}C{u_n}{O_{2n + 3}}\] and in the \[TlB{a_2}C{a_{n - 1}}C{u_n}{O_{2n + 4}}\] compound, it decreases after three \[Cu{O_2}\] layers.
\[Hg - Ba - Ca - Cu - O\] Superconductor:
-
The crystal structure of \[HgB{a_2}Cu{O_4}\] ( \[Hg\] -1201), \[HgB{a_2}CaC{u_2}{O_6}\] ( \[Hg\] -1212) and \[HgB{a_2}C{a_2}C{u_3}{O_8}\] ( \[Hg\] -1223) is similar to that of \[TL\]-1201, \[TL\]-1212 and \[TL\]-1223, with in place of \[TL\] .
-
It is noteworthy that the \[{T_c}\] of the Hg compound (\[Hg\]-1201) containing one \[Cu{O_2}\] layer is much larger as compared to the one- \[Cu{O_2}\] -layer compound of thallium (\[TL\]-1201).
-
In the \[Hg\] -based superconductor, \[{T_c}\] is also found to increase as the \[Cu{O_2}\] layer increases.
-
For \[Hg\]-1201, \[Hg\]-1212 and \[Hg\]-1223, the values of \[{T_c}\] are 94, 128 and the record value at ambient pressure 134 K, respectively.
Table-1 High temperature superconductors
|
Formula |
Notation |
Tc (K) |
No. of Cu-O planes |
Crystal structure |
|
\[YB{a_2}C{u_3}{O_7}\] |
123 |
92 |
2 |
|
|
\[B{i_2}S{r_2}Cu{O_6}\] |
Bi-2201 |
20 |
1 |
|
|
\[B{i_2}S{r_2}CaC{u_2}{O_8}\] |
Bi-2212 |
85 |
2 |
Tetragonal |
|
\[B{i_2}S{r_2}C{a_2}C{u_3}{O_6}\] |
Bi-2223 |
110 |
3 |
Tetragonal |
|
\[T{l_2}B{a_2}Ca{O_6}\] |
Tl-2201 |
80 |
1 |
Tetragonal |
|
\[T{l_2}B{a_2}CaC{u_2}{O_8}\] |
Tl-2212 |
108 |
2 |
Tetragonal |
|
\[T{l_2}B{a_2}C{a_2}C{u_3}{O_{10}}\] |
Tl-2223 |
125 |
3 |
Tetragonal |
|
\[TlB{a_2}C{a_3}C{u_4}{O_{11}}\] |
Tl-1234 |
122 |
4 |
Tetragonal |
|
\[HgB{a_2}Cu{O_4}\] |
Hg-1201 |
94 |
1 |
Tetragonal |
|
\[HgB{a_2}CaC{u_2}{O_6}\] |
Hg-1212 |
128 |
2 |
Tetragonal |
|
\[HgB{a_2}C{a_2}C{u_3}{O_8}\] |
Hg-1223 |
134 |
3 |
Tetragonal |
Properties of High Temperature Superconductors.
-
HTS are brittle in nature
-
The properties of the normal state of these materials are highly anisotropic.
-
The Hall Coefficient is positive indicating the charge carriers are hole.
-
Their behavior can’t be explained by BCS theory.
-
The isotopic effect is almost absent in these materials.
-
The magnetic properties of these materials are highly anisotropic.
-
The effect of pressure is different on different materials e.g the application of pressure increases the critical temperature of \[LBCO\] compounds but decreases the critical temperature of \[YBCO\]
Application of HTSC
Commercial quantities of HTSC wire based on \[BSCCO\] are now available and applications include transformers, fault current limiters, power storage, motors and fusion reactors etc.
HTSC cables-
Lower voltage at the same power rating
Low impedance power guiding
Lower life cycle costs
No soil heating
No electromagnetic spray fields.
Electrical Machines-
High quality of generated power
Improved voltage stability
More reactive power is available at a given rating
Low total harmonic distortion
Low maintenance
Size and Weight reduction
High efficiency and low operating cost
Fault current limiters-
Over 100times faster response time
Time adjust response function
Full power protection cycles
Magnets-
Higher operational temperature range up to 77 K
Compact and high weight
Higher magnetic field of the order of 100 Tesla
Greater stability and thermal efficiency
Less complex cryogenic system
Higher signal to noise ratio
Transformers-
- HTS turns power transformer into compact, highly efficient and environment friendly performers.
RF and Microwave Filters-
Medical Diagnosis-