Site pages
Current course
Participants
General
Module- 1. Introduction of food plant design and ...
Module- 2. Location and site selection for food pl...
Module- 3. Food plant size, utilities and services
Module- 4. Food plant layout Introduction, Plannin...
Module- 5. Symbols used for food plant design and ...
Module- 6. Food processing enterprise and engineer...
Module- 7. Process scheduling and operation
Module 8. Building materials and construction
19 April - 25 April
26 April - 2 May
Lesson 15. Building materials
15.1 Introduction
The building materials used vary considerably in different parts of the world. Though, basic materials used for the construction of food plant are common in almost all countries. More emphasis has been given for the materials which are commonly used in India. The use of cement, bricks, cement concrete, RCC, steel, stone, wood, metal, glass etc. are very common in most countries. In recent years, pre-fabricated blocks and pre-stressed RCC blocks are being employed for various applications. It is necessary to consider several factors such as environmental conditions in the plant, whether conditions, wear and tear, effect of acid and alkalis, safety, cost etc. for the selection of materials for different sections of food plants. There are different types of stones used for flooring and skirting and it should have desirable properties to with stand to prevailing conditions of different sections. Similarly requirement of type of wood depends on its position in a windows, doors and other applications, soft woods requires seasoning treatment and application varnish and oil paints to prevent yeast and mould growth. Selection of metal depends on type of vessels used for processing, particularly to with stand high pressure and to meet sanitary standards. The glasses used in the plant are specifically designed with wire mesh inside, so that no glass pieces will fall in the product. In certain sections like, butter, ghee cheese and fruit/vegetable product manufacturing, glasses are used to control sun light particularly UV light to prevent oxidation of fat and related defects in the product.
15.2 Factors to be considered for selection of building construction materials
-
Effect of atmospheric conditions on the building
-
Effect of prevailing conditions in the plant such as water, water vapor, high temperature, acids, alkalis and wear and tear.
-
Durability of material under conditions to which it is exposed
-
Ease of cleaning and maintenance
-
Availability of the materials
-
Cost of materials
15.3 Properties of building materials
The properties of different building materials are discussed in 15.4. Some general properties which are to be considered are discussed here. The use of building materials of organic origin which may be attacked by bacteria and other organisms should be avoided. Whenever soft wood and wood based products are necessary, protection against germs and moisture is very essential requirement. Many hardwoods such as teak have shown good serviceability for use of window frames, doors etc.
Insulating materials must be well protected to prevent entry of water vapour. The use of PUF panels for insulation of cold storage has changed the entire construction requirement of cold storages. The use of paints and bonding materials of organic origin should be avoided. The building materials are exposed to corrosion environment in dairy plant must be protected. Iron under moist condition rusts quickly and hence it must be protected by galvanizing, zinc coating or painting. The condensation of water vapor on a wall or pipelines may cause corrosion of metals and seriously damage the paint. Therefore, removal of water vapour from the processing room be taken into account.
15.4 Building materials
Generally building materials are classified into three groups as under.
(i) Solid materials – stones, bricks, iron etc.
(ii) Cement Materials – lime, cement etc.
(iii) Protective materials – paints, varnishes etc.
15.4.1 Bricks
Clay is the basic raw material for the preparation of bricks. Clay for brick is composed of alumina and silica and certain fluxing ingredients such as ferric oxide, lime, magnesia or CO2 in quantities up to 20% except in firebrick, which may have silica content as high as 98%.
Bricks are obtained by moulding clay in rectangular blocks and then drying and burning of those blocks. It is manufactured by many places throughout the country in unorganized sectors. Brick is widely used as building material for making walls and as compared to other materials used for this purpose, it is cheaper. Brick contains alumina, silica, lime, oxide of iron, magnesia etc. and each constituent imparts specific characteristics in brick.
15.4.1.1 Properties of Brick
Good quality brick should be well-burnt in kilns, uniform in size and colour, clear metallic ringing sound when struck with each other.
No brick should have crushing strength below 5.50 N/mm2. The bricks should not break into pieces when dropped flat on hard ground from a height of 1 m.
Absorption of water should be more than 20% of its weight in 16 h immersion in water.
Crushing strength: As per BIS, minimum crushing strength of brick is 3.50 N/mm2
7 to 14 N/mm2 : A grade; 14 N/mm2 : AA grade
Hardness: Bricks should be sufficiently hard.
Presence of soluble salt: bricks should possess minimum salts.
Shape and size: Rectangular shape with sharp edges. (Size: 190 mm x 90 mm x 90 mm (recommended by BIS)
Soundness: Clear ringing sound when two bricks are struck with each other.
Structure: homogeneous, compact, free from any defects like holes, lumps.
15.4.1.2 Constituents of good brick
(i) Oxides of aluminum (Al2O3): This ingredient renders the plastic property in the material required for moulding. However, excess of it causes the raw brick to shrink & wrap, while drying.
(ii) Silica or sand (SiO2): It prevents the raw bricks from warping, shrinking and cracking. But excess amount of silica may cause brittleness.
(iii) Lime (CaO): Lime helps in preventing the shrinking of the raw bricks. A small proportion of lime present in a finely divided state also acts as a flux and causes the sand to fuse and bind the particles to gather. Excess of lime causes the brick to melt and run out of shape. Lumps of lime become quick lime after burning and when the brick is immersed in water or absorbs moisture from the air, the quick lime slakes, expands and causes the brick to split into pieces.
(iv) Oxides of iron (Fe2O3): A very small percentage of oxide of iron is helpful in (a) causing the sand to fuse a little at low temperature giving a pleasing tint to burnt brick.
(v) Magnesia (MgO): It gives the brick a yellow tint colour, if present in small quantities.
15.4.2 Fire brick
Fire bricks are very important for high temperature industrial applications. The materials which are capable of resisting high intensity of heat i.e. silica, alumina, magnesite, bauxite and chromite are added in fire brick. The composition of a good fire clay suitable for fire brick is 50-70% silica, 10-25% alumina, 2-2.5% iron oxide or lime magnesia, < 1.5% alkali.
15.5 Sand
It consists of small grains of silica (Si02). It is formed by decomposition of sandstones due to weather effects.
15.5.1 Types of sand
Sands available in market are classified based on the source as under.
(i) Pit sand
(ii) River sand
(iii) Sea sand
Based on the size of the sand, it is classified as under.
· Fine sand: It should pass through 1.59 mm opening (suitable for plastering)
· Course sand: It should pass through 3.18 mm opening (suitable for masonry work)
· Graveled sand: It should pass through 7.62 mm opening (suitable for concrete work)
15.5.2 Desired properties of sand
Objectives of mixing sand to prepare mortar are:
To prevent excessive shrinkage.
To improve the strength of mortar.
To improve the setting power. Sand makes the mortar porous, which absorbs CO2 from air and becomes hard.
To increase the bulk and thus reduce the cost
The sand used for making mortar should be clean, coarse, hard, free from any clay, dust, mica particles and soft flaky pieces. Sand required for brick work needs to be finer than that for stone work.
15.6 Lime
The use of lime as cementing materials has been made since ancient times, but at present cement has replaced lime to a great extent. Lime is produced by calcinations of limestone.
The lime which is obtained by calcinations of comparatively pure limestone is known as quick lime. It is made in kilns. Addition of water (about 32% of CaO) to the quick lime to prepare hydrate lime is called slaking of lime.
![]()
A thin suspension of slaked lime is known as the milk of lime
15.6.1 Use of lime
Mortar for masonary work
White washing
Purification of water and sewage treatment
Many industrial application
15.7 Limestone
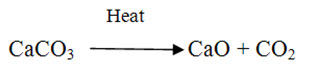
Limestones (CaCO3) either in pure or mixed with impurities is the parent material from which lime and cements are produced. Limestone is inert and insoluble in water in its natural condition. When limestone is burnt in kiln or calcined, CO2 is separated and driven out in the form of gas and what remains is CaO in the form of lumps, called quick lime, which is soluble in water. When water is sprinkled on quick lime, it breaks in to powder, which is Ca(OH)2 known as hydrated lime. The process is called slaking.
15.8 Cement
The ordinary cement was invented by a Joseph Aspdin in England in the year 1824. The inventor took a patent and called it Portland cement as it resembles in its colour after setting to a variety of stone found in Portland. The raw materials for cement are (i) lime stone (ii) coal (iii) clay and (iv) gypsum. Cement can be made by two different processes (i) dry and (ii) wet process.
15.8.1 Composition of ordinary/Portland cement:
Lime (CaO) : 62%
Silica (SiO2) : 22%
Alumina (Al2O3) : 5%
Calcium sulphate (CaSO4) : 4%
Iron oxide (Fe2O3) : 3%
Magnesia (MgO) : 2%
Sulpher (S): 1%
15.8.2 Function of cement ingredients:
The properties of cement depend upon its composition, burning treatment and fineness of grinding. Good quality cement should provide strength, binding and water resistant in addition to working, quick setting and plasticity during construction work. The role of different constituents of Portland cement is presented below.
Lime: It provides strength and setting property but excessive lime makes the cement unsound and causes the cement to expand and disintegrate. Its deficiency causes less strength and quick setting.
Silica: It imparts strength due to formation of dicalcium and tricalcium silicate. The excess of silica improves strength but causes prolonged setting.
Alumina: It imparts quick setting property and acts as flux. The excess of it weakens the cement.
Calcium Sulphate: This ingredient is in the form of gypsum and its function is to increase the initial setting time of cement.
Iron oxide: It imparts colour, hardness and strength to the cement.
Magnesia: It imparts hardness and colour. The excess of it makes the cement unsound.
Sulpher: A very small amount of sulpher is useful in making cement sound. The excess of it causes cement to become unsound.
Alkalies: The excess quantity causes staining in concrete.
15.8.3 Setting action of cement
When water is added to cement, the ingredients of cement react chemically and form various chemical compounds. The mixture goes on thickening till it achieves a rock like state. It is found that ordinary cement achieves about 70% of its final strength in 28 days and about 90% in one year.
The important compounds formed during setting action of cement are as under.
· Tricalcium aluminate (3CaO. Al2O3): It forms within 24 h after addition of water to the cement.
· Tetra-calcium alumino ferrite (4CaO. Al2O. Fe203): It forms within 24 h after addition of water.
· Tricalcium silicate (3CaO. SiO2): It forms within a week after addition of water to the cement. It is responsible for imparting strength in early period of setting.
· Dicalcium silicate (2CaO. SiO2): It forms slowly and imparts progressive strength.
The above 4 compounds in Portland cement are designated in short as C3A, C4AF, C3S and C2S respectively.
15.8.4 Types of Cement
15.8.4.1 Rapid hardening cement/ high early strength cement:
The setting time of this cement is the same as that of the ordinary cement. The high strength at early age is due to finer grinding, burning at higher temperature and increased lime content in the composition. This cement costs a little more than ordinary cement. The strength developed by this cement in four days equals that acquired by ordinary cements in 28 days. Thus, it is very important in increasing the speed of construction.
15.8.4.2 Quick setting cement
Under normal conditions, quick setting is considered as a defect, as it does not allow sufficient time for the concrete to be properly mixed and placed. The addition of 3 to 4 % of gypsum is added to the ordinary cement just to retard the setting action. But under certain conditions, when concrete is to be laid under water or in running water, quick setting cement is required. Fineness of grinding and the addition of a small percentage of aluminium sulphate accelerate the setting action. The setting action of such cements starts within 5 minutes after addition of water and it becomes stone hard in less than half an hour.
15.8.4.3 High Alumina cement:
This is manufactured by melting a mixture of bauxite and lime and grinding the resulting clinkers. Total alumina content shall not be less than 32% and the ratio by weight of alumina to lime shall not be less than 0.85 nor more than 1.3. It is not only rapid hardening cement, but has also higher ultimate strength. It gives 6000 psi compressive strength against 2500 psi compressive strength obtained in 7 days for Portland cement. Its initial setting time is more than 3.5 hours and final setting time is 4 to 5 hours as against 0.5 hours and 10 hours respectively in case of ordinary cement. It therefore allows longer time for mixing and placing concrete before it begins to set.
High Alumina cement is immune to thermal shocks and great heat is evolved during setting and hardening. It does not expand on setting. It possesses great resistance to corrosion action of acid and also to high temperatures. Thus it is found very useful in chemical plants, mines, dairies etc. and also for lining the furnaces. It is also high resistant to sea water.
15.8.4.4 Acid resistance cement
The addition of additives like sodium fluosilicate accelerates the hardening process and it increases the resistance to acid and water. It also contains acid resistant aggregates.
15.8.4.5 Coloured cement:
The cement of desired colour may be obtained by mixing pigments with ordinary cement. The amount of colouring material may vary from 5-10%. The use of chromium oxide and cobalt pigments imparts green colour and blue colour respectively while iron oxide and manganese oxide gives brown/red colour and black or brown colour respectively.
15.8.4.6 White cement
It is prepared without colouring oxides. The process of making the cement is slightly different as coal is not used for burning. White cement is used for fixing marbles, filling of joints in flooring and glazed tiles. It is relatively costly as compared to Portland cement.
15.9 Mortar
The paste prepared by adding required quantity of water to a mixture of binding material like cement and sand is known as mortar. The proportion of cement to sand varies between 1:2 and 1:6 depending on the use of mortar. It is desirable to use the mortar within 30 minute after addition of water. After setting of mortar, it should be kept damp or wet by sprinkling water to avoid drying of mortar for about 7 to 10 days.
15.9.1 Properties of mortar
The properties of good mortar are listed below.
· Good adhesion with the building.
· Capable of developing required stresses.
· Capable of resisting penetration of water
· Durable
· Good workability-mobility
· Good placeability
15.9.2 Uses of mortar
Binding material for building materials (bricks, stone etc.)
Plaster work
Bedding layer for building units
Joining of cement pipes, filling of cracks.
15.10 Cement concrete
The cement concrete is a mixture of cement, sand, pebbles or crushed rock and water which when placed in the skelton of forms and allowed to cure, becomes hard like a stone. The cement concrete in which steel reinforcement is placed at suitable places to increase tensile stress is called Reinforced Cement Concrete (RCC). The proportion of cement, sand and course aggregates varies from 1:2:4 to 1:3:6 depending on the nature of work. As per BIS, the concrete is designated in 7 grades. These grades are M 10, M 15, M 20, M 25, M 30, M 35, M 40 (M refers the mix and number indicates the compressive strength of 28 days in N/mm2). The following points are important for R.C.C.
< M 15 should not be used in R.C.C. work
Water-cement ratio by weight should be 0.45 –0.55
Weight of water = 28-30% weight of cement + 4-5% weight of total aggregate.
Thickness of R.C.C. work : 80 to 150 mm
Proper mixing of all materials – using mechanical mixer is very essential.
Placing of concrete within 30 minutes
Consolidation using vibrator helps in reducing air bubbles and increases the strength of R.C.C . Hand consolidation which includes ramming, tamping, spading, slicing with suitable tools.
Provide expansion and contraction joints if length exceeding 12 m.
Dummy joint – 3 mm width and 1/3 to 1/5th of slab thickness in depth and it is filled with filler materials.
15.10.1 Curing of concrete
The concrete surface is kept wet for certain period after placing of concrete so as to promote hardening of the mixture. Curing period is about 7 to 14 days. Ponding with water, covering with wet jute bags, intermittent spraying with water etc. may be used for curing.
15.10.2 Water proofing cement concrete
The impermeability of concrete is very essential. If concrete is made dense and free from cracks, it is watertight. This can be achieved by closely adhering the following points.
Using high class Portland cement
Adopt correct proportioning of sand, cement and aggregate.
Using clean and non-porous aggregates
Proper mixing at optimum water quantity.
Careful placing, tamping and curing.
Using suitable water proofing compound.
15.11 Stones
Stones are obtained from rock, a portion of the earth’s crust having no definite shape or chemical composition. Stones are used as the construction material for the following.
Material for foundations and walls of buildings, dams, bridges
Material for road and concrete making in the form of either broken or crushed stones called aggregates/ gravels/ peebles.
Used as thin slabs for paving/flooring.
Used as roofing tiles
Lime stones are used as a flux material in the blast furnaces
In view of several artificial materials developed recently for flooring and roof, the use of natural stone in many applications has become limited.
15.11.1 Requirements of good structural stones
When stone is used as structural material, the following considerations are to be considered.
(i) Strength: It should be sufficiently strong against crushing. The stones having more compact grains with higher density are stronger. A dense and compact stone has very few or no pores and thus does not absorb water. A stone of igneous origin is stronger than one of sedimentary formation. A crystalline stone is superior to a non-crystalline one and the finer the crystalline structure, the stronger it is. The specific gravity of good stone should not be less than 2.7. It is desirable to have crushing strength of building stone more than 100 N/mm2. The crushing strength of some commonly used stone is given below.
· Granite: 75-127 N/mm2
· Sand stone: 64 N/mm2
· Lime stone: 54 N/mm2
· Durability: It depends on (a) chemical composition (b) physical structure (c) weathering effects and (d) place or position in the structure. It depends on type of stone and working conditions.
· Appearance: The appearance of a stone in relation to the design is of great importance from an architectural point of view. Appearance depends on the colour and the ease with which stone can be dressed, rubbed or polished, for which slightly softer stone is selected. It is desirable that the colour of stone is uniform, no weathering effect, and free from clay holes.
· Dressing properties: The ability of stone for cutting, carved etc is important for building material. In order to perform dressing operations with ease, it should be relatively soft with compact grains. Stone should be homogeneous in texture rather than crystalline. The development of many machines and advancement of technology, the dressing of even hard stone has become quite easy for many applications of stones.
· Hardness: It is expressed in terms of co-efficient of hardness. The common accepted norms for various categories of hardness are greater than 17 considered as high, between 14 to 17 as medium hardness and less than 14 as poor hardness.
· Wear percentage: If the value of wear is more than 3 percent, then the stone is regarded as unsatisfactory.
· Seasoning and Weathering: After quarrying stone should be seasoned for 6-12 months and there should not be any change in various properties. A good quality of stone should not show remarkable weathering effect.
· Texture: Stone should be free from cavities, cracks etc.
· Toughness: It is expressed in terms of toughness index. The common accepted norms for various categories of toughness are greater than 19 considered as high, between 13 to 19 as moderate toughness and less than 13 as poor toughness.
· Water absorption: The absorption of water should not exceed 0.6 % of weight.
15.11.2 Basis of classification of stones
There are 4 basis of classification of stones as under.
1) Geological (based on mode of formation)
2) Physical (depending upon structure)
3) Chemical (depending on composition)
4) Practical (based on use)
15.11.3 Use of stone
Use of natural stone is various purposes is given below.
(i) Structure (ii) Face work
(iii) Paving (iv) Basic material
15.11.4 Stone Quarrying
The process of taking out stone from natural rock is knows as quarrying.
15.11.5 Artificial stones
Cement concrete blocks
Mosaic tiles
Terrazo
Ceramic tiles
15.12 Wood
Wood is of two types – hard wood and soft wood. Hard wood is used for building construction and soft wood is mostly used for packaging material and boxes. Plywood is obtained from inferior quality wood after some processing, and it is used for making furniture. Hardwood is seasoned to reduce moisture content and is also treated with creosote oil. Hard woods are used in the room where moist conditions prevail and it is properly seasoned to prevent any kind of mould growth and contamination. Soft woods are used in the dairy for cold store doors and covered with stainless steel sheet.
Soft wood is obtained from trees of Deodar, Kali, Chir, Pine, Walnut and Spruce etc. Soft woods are resinous and light in colour. It is general characteristics that trees having needle like leaves give soft wood.
Sal, Teak, Shisham, Pyngado, Oak, Beach and Ash are some of the examples of hard wood obtained from broad leaf trees. Hard woods are relatively darker in colour, heavy, close grained and strong. They are non-resinous.
15.12.1 Timber wood
Timber wood is suitable for building or engineering purposes. When in living tree, the timber is called ‘standing timber’. When trees are cut down, it is called ‘rough timber’ and when it is sawn into various market sizes, such as beams, battens, posts, planks etc., it is called ‘convert timber’
15.12.2 Seasoning of wood
The growing tree contains a large amount of moisture which may be 150% of the dry weight of the timber. The process of removal by drying the excess moisture from the wood in a controlled manner to prevent the shrinkage which occurs, causing cracks and other defects is called seasoning. Seasoning may be natural or artificial. Natural seasoning is best but it takes long time. Artificial seasoning may be water seasoning, boiling or kiln seasoning.
15.12.3 Preservation of wood
Timber is liable to attack by dry rot and other fungi. The function of a preservative is to poison the food matter in the timber. But this poison should not be dangerous to carpenter and must not wash out in rain. The best time to apply preservatives is the early summer. The usual methods of preservation are tarring, charring, painting, creosoting, solignum paints and Ascu treatment.
Tarring consists of coating the timber with hot coal tar. Tarring is adopted only for work of rough character such as timber fences, ends of doors and window frames built into walls.
Charring is adapted to the portions which are embedded in the ground. The ends of posts are charred over a wood fire to a depth of about ½” and then quenched with water. Painting consists of applying 3 or 4 coats of an oil paint. Solignum paints are effective preservatives against attack by white ants. The process of Ascu treatment for the preservation of wood has been developed at the Forest Research Institute at Dehradun. The powder developed for the purpose is dissolved in water. Six parts of powder are dissolved in 100 parts of water and then applied or sprayed on timber.
15.12.4 Fire proofing
Wood can catch fire very easily and it is difficult to make it fireproof. However, chemicals such as 2 coats of 2% solution of Borax or sodium arsenate are effective in retarding the action of fire.
15.13 Metals
Metals are used in the dairy for different purposes. Mild steel is used for fabrication of sheds, grills, foundations, pipelines, and as structural steel. Cast iron is used for drainage of effluent as well as for the fabrication of various components of machines. Stainless steel is used for fabrication of dairy equipment and milk pipelines.
15.13.1 Stainless steels (SS)
Stainless steels were invented to overcome the problem of corrosion which is a major concern of food and many other industries. The alloy of steel containing iron-chromium-nickel is known as stainless steels which do not rust in sea water and are resistant to acids and several other chemicals. Stainless steels typically contain between 9 and 30 percent chromium and varying amounts of nickel, molybdenum, copper, sulfur, titanium, niobium, etc., may be added to obtain the desired mechanical properties and service life. Having all these properties, SS are widely used in dairy and food industry. Stainless steel is considered noble metal for use in dairy industry. Stainless steels are classified based on the chemical composition and it provides information to overcome many types of corrosion. Some of the limitations of SS employed in food and dairy industry are attack by lactic and malic acids at elevated temperature and poor thermal conductivity. However, these limitations may be overcome by carefully selection & fabrication, optimized operating condition, care and maintenance of the equipment.
15.13.1.1 Classification of SS
Stainless steels are basically classified as austenitic, ferritic, martensitic, duplex and super-austenitic grades. Each of these main groups contains a number of alloys that are defined according to the chemical composition and specified in European and American international standards. Apart from chromium, the alloy constituents molybdenum, nickel and nitrogen are of great importance to the corrosion resistance. Carbon will always be present to a certain degree, and it is important for the welding properties. In addition, copper, manganese, sulphur, titanium and niobium are used as alloy constituents to impart certain properties. Stainless steel is typical wrought alloy AISI (American Iron and Steel Institution) series designations, includes: 200 (high manganese austenitic), 300 (austenitic), and 400 & 500 (ferritic & martensitic). Martensitic and ferritic steels are magnetic and martensitic steels are typically hardened by heat treatment and are not easily formable. Austenitic steels harden when cold worked. Duplex grades (austenitic/ferritic) are more resistant to stress corrosion cracking than austenitic and are tougher than ferritic grades.
15.13.1.2 Desirable properties of SS
The properties of stainless steel play an important role in the design of various equipments. The use of high quality SS in fabrication of processing equipments helps not only to prevent corrosion but also ensures purity of food product handled in those equipments. In addition to this, stainless steels are easy to clean and maintain and a number of different products can be manufactured in the same equipment. If properly utilized, equipment made of stainless steel can be expected to last for many years. In selecting austenitic stainless steels, a number of factors other than corrosion performance should be considered. Among these are their usually attractive appearance, good mechanical properties, and excellent fabrication characteristics. On a life-cycle basis, the alloys are often the most cost-effective. Important characteristic to be considered in selecting the proper type of stainless steel for a specific application are listed below.
· Corrosion resistance
· Resistance to oxidation and sulfidation
· Strength and ductility at ambient and service temperatures
· Suitability for intended fabrication techniques
· Suitability for intended cleaning procedures
· Stability of properties in service
· Toughness
· Resistance to abrasion, erosion, galling, and seizing
· Surface finish and/or reflectivity
· Physical property characteristics such as magnetic properties, thermal conductivity and electrical resistivity
· Total cost, including initial cost, installed cost, and the effective life expectancy of the finished product
15.13 Steel
Steel is an intermediate stage between cast iron and wrought iron. The cast iron contains 2-4 % carbon while wrought iron has carbon content less than 0.15%. In steel, the carbon content varies between 0.25 to 1.5 %. There is no graphite in the steel. The steel becomes harder and tougher as its carbon content increases. Steel is used for various applications in dairy industry.
15.14 Aluminium
Aluminium is used as frame work for doors and windows in dairy and food processing plants. The desirable properties of aluminium which makes it suitable are light weight, softness, and appearance. Its use is limited by the fact that it is tarnished and corroded by ordinary alkaline dairy cleaners and sterilizers.