Site pages
Current course
Participants
General
Module 1: Basics of Agricultural Drainage
Module 2: Surface and Subsurface Drainage Systems
Module 3: Subsurface Flow to Drains and Drainage E...
Module 4: Construction of Pipe Drainage Systems
Module 5: Drainage for Salt Control
Module 6: Economics of Drainage
Keywords
12 April - 18 April
19 April - 25 April
26 April - 2 May
Lesson 14 Reclamation of Chemically Degraded Soils
14.1 Overview of Salt-Affected Soils and Acid Soils
14.1.1 Classification of Salt-Affected Soils
Salt-affected soils are generally classified as saline, alkali or saline-alkali, based on the values of EC, ESP and pH of the soil saturation extract. These classes are defined as follows (Bhattacharya and Michael, 2003):
(i) Saline Soil: ECe > 4 dS/m, ESP < 15% and pH < 8.5.
(ii) Alkali Soil: ECe < 4 dS/m, ESP > 15% and pH > 8.5.
(iii) Saline-Alkali Soil: ECe > 4 dS/m, ESP > 15% and pH = 8.5 or slightly higher.
The above classification of salt-affected soils help to identify whether salinity is the major constraint to agriculture or soil alkali condition or both salinity and alkali condition which are adversely affecting the plant growth and crop yield. Based on such understanding, appropriate remedial measures can be selected for the reclamation of lands having above types of soils or for the selection of crops which can survive and yield reasonably well even under such adverse soil conditions.
In general terms, and from agricultural viewpoint, the soils of all the above classes can be considered as chemically degraded because of poorer crop performance in such soils compared to that in the normal soils (Bhattacharya and Michael, 2003). Other types of chemically degraded soils are those which have too poor soil fertility to support plant growth and which are polluted by human activities such as dumping of untreated industrial and mine wastes containing toxic chemicals in streams or on the land. Nutrient availability to the plant is dependent upon the degree of salt problem. Usually, salt-affected soils need higher doses of fertilisers to meet the plant requirement. Plant roots experience difficulty in extracting water from the soil if the concentration of the dissolved salts in the soil solution is high, which raising its osmotic pressure. This is why, in highly saline soils, despite having adequate soil moisture, plants may show wilting symptom similar to the situation when there is a lack of adequate soil moisture (Bhattacharya and Michael, 2003). Intensive agriculture, supported by irrigation may also lead to chemical degradation of soil through the depletion of nutrients and the rise of water table (waterlogging).
14.1.2 Acid Soils
Apart from salt-affected soils, a special type of degraded soil is acid soil. Acid soil is defined as a soil having pH of its saturation extract less than 7. Acid soils are also considered degraded because plant growth and crop production are directly and seriously hampered under low pH conditions of the soil solution and indirectly through lower nutrient uptake capability of the plants in the acidic root zone environment. Fortunately, the severely acidic soils occupy much smaller area in India as compared to the severely salt-affected soils (Bhattacharya and Michael, 2003). Also, unlike alkali soils, a highly acidic soil does not remain so throughout the year because the rains during the rainy season dilute the acids considerably. This is particularly true in monsoon-dominated climatic regions.
An important reason of soil acidity is the oxidation of Sulphur compounds in the soil under the action of atmospheric Oxygen and eventual conversion of the SO2 into H2SO4 with the addition of water. Sulphur and its compounds may be present in the soil or may have been dumped along with the wastes while extracting metal ores from mines (ores of Copper, Lead, Iron and Zinc mainly occur in their Sulphide form) or may have come due to land inundation (as in the case of the coastal Kerala) by seawater which contains H2S (Bhattacharya and Michael, 2003). Under this situation, the acidity may be very severe having soil pH as low as 4 or even lower.
Acid soils are also found in the lateritic regions of India such as in West Bengal, Bihar, Odisha, Goa and Karnataka. Acidity due to high rainfall as the major factor is not severe because pH is normally above 5, with some exceptional locations where it may be lower than this. Besides the causative factors of the oxidation of Sulphur compounds and local rainfall behaviour, soil acidity is also caused due to the acidic nature of the some of the constituents of the parent material of the soil. In general, acid soils are found in the north, north-east, east (including the coastal region), south and west coastal regions of India (Bhattacharya and Michael, 2003). The most severely acidic soils are found in the low-lying (below sea level) bunded rice fields in the Kuttanand region of Kerala, where pH as low as 3.2 has been reported (Mathew et al., 1993). The states like Punjab, Uttar Pradesh, major parts of Bihar and Madhya Pradesh, Rajasthan, Gujarat an Maharastra do not suffer from the problem of soil acidity. A comprehensive discussion on the occurrence, genesis, nutrient status, crop response and management of acid soils of India is given in Anonymous (1976). A brief description of the reasons for forming acid soils, their types and the economic feasibility of acidity amelioration is presented by Panda et al. (1996).
The commonly used method of neutralizing soil acidity is the application of lime. This is routinely practiced by the rice farmers of the Kuttanand region in Kerala. Such chemical amendment, supplemented by subsurface drainage and application of suitable doses of nutrients, has been found to improve rice productivity to a large extent.
14.1.3 Distribution of Chemically Degraded Soils in India
Abrol and Gupta (1991) provided an estimate of total salt-affected soils in India, as shown in Table 14.1. This table shows that the total estimate of salt-affected soils is 7.044 Mha. The geographical distribution of sodic soils, inland saline soils and coastal saline soils in the Indian mainland is shown in Fig. 14.1 (Abrol and Gupta, 1991).
Table 14.1. Distribution of salt-affected soils in the Indian mainland (Source: Abrol and Gupta, 1991)
|
Sl. No. |
Category |
States |
Area (Mha) |
|
1 |
Arid and Semi-Arid Regions |
Gujarat, Haryana, Punjab, Rajasthan, Uttar Pradesh |
1.0 |
|
2 |
Indo-Gangetic Plains |
Bihar, Haryana, Madhya Pradesh, Punjab, Rajasthan, Uttar Pradesh |
2.5 |
|
3 |
Medium and Deep Black Soil Regions |
Andhra Pradesh, Gujarat, Karnataka, Madhya Pradesh, Maharashtra |
1.42 |
|
4 |
Coastal Regions: |
||
|
Gujarat |
0.714 |
|
|
Andhra Pradesh, Odisha, Tamil Nadu, West Bengal |
1.394 |
|
|
Kerala |
0.016 |
|
|
Total = 7.044 |
|||
A relatively recent report (Singh, 1994) mentions the salt-affected area in India as about 8.57 Mha, comprising about 3.47 Mha in various canal command areas, 3.03 Mha in non-canal command areas and 2.07 Mha in the coastal regions. This report also mentions that the area under acid soil having pH < 6.5 is 49 Mha, of which the area having pH < 5.5 is 26 Mha. Another study (Suraj Bhan, 1995) reports the total salt-affected soils (including coastal saline soil) as
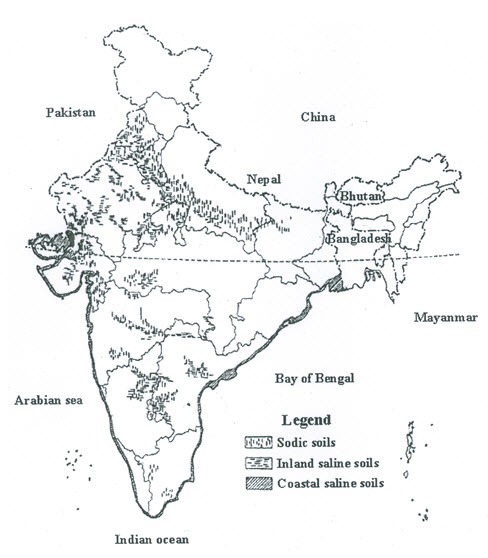
Fig. 14.1. Approximate distribution of salt-affected soils in India. (Source: Abrol and Gupta, 1991)
7.028 Mha and acid soils as 0.016 Mha. It should be mentioned that soil acidity gets diluted during rainy seasons. With the addition of chemical amendments and drainage, such soils are capable of giving good rice yield in high rainfall regions where rice is a preferred crop. In addition, certain plantation crops, especially tea, prefers an acidic soil environment with a pH 4.5 and 5.8 (Rehman, 1991). Such lands, though degraded based on the pH level, are not constraints to agricultural production.
14.2 Reclamation of Saline Soils
14.2.1 Introduction
Reclamation of chemically degraded soils is based on the concept of removal (leaching) of dissolved salt solutions from the root zone. In case of saline soils, addition of adequate quantity of water and its percolation through the soil dilutes the soil water in the soil profile and pushes them downward; this process is called leaching. If water table is shallow or natural subsurface drainage is restricted, the percolated soil solutions are to be removed by installing a subsurface drainage system. Even so, the permanent reclamation may be difficult because the hot and dry weather prevailing over several months (particularly in the monsoon climate as in India) resalinizes the soil profile. In the costal region, there may be considerable upward flux of saline groundwater from below and that would also require long-term functioning of the subsurface drainage system to continuously remove salts through leaching. Fortunately, a well-designed and executed subsurface drainage system has a long life and the leaching requirement is automatically taken care as long as freshwater is used for irrigation and leaching is practiced (if necessary).
14.2.2 Estimation of Leaching Requirement
(1) Theoretical Estimation
In the most simple way, leaching requirement (LR) is the difference between the deep percolation from the root zone and the capillary flow from groundwater to the root zone, both expressed in units of depth of water over a certain period (e.g., mm/day, mm/month and so on). Under irrigation or rainfall, the outflow from the root zone is always greater than the inflow into the root zone due to upward capillary movement of water from deeper layers. Hence, LR is a positive quantity. As mentioned in Lesson 13, LR can also be expressed as a fraction of irrigation water that must be deep percolated to maintain a desirable salt concentration in the root zone (i.e., neither depletion nor build up of salt). For the reclamation of saline soils, the salt content of the root zone should be depleted. Therefore, conceptually the LR can be defined as the amount of water that has to move down beyond the root zone to maintain a favorable salt balance in the root zone.
Hypothetically, if one assumes that the applied irrigation water has negligible EC and further assumes that the added irrigation water act like a piston in pushing down the saline water in a saturated soil, then the leaching requirement becomes simply the depth of water required to saturate the soil to a given depth. If this happens, then a saline soil could be reclaimed by leaching with minimum possible quantity of added irrigation water and the existing soil salinity would reduce to zero or to a negligible value due to such a hypothetical leaching. In reality, however, such type of irrigation water quality is not available in practice; even rainwater has a measurable EC in the range of 0.1 to 0.2 dS/m. Moreover, a piston like water movement does not take place through the soil profile. While some of the added water first saturates the soil, some portion moves down faster through larger capillary pores and cracks, particularly in unsaturated soil comprising swelling and shrinking type of clays. The water that moves down faster, does not get an opportunity to thoroughly dilute the salt solutions in the soil pores, and hence does not perform proper leaching. The part of the added water that moves through the soil pores mixes with the saline soil water and the solution moves down under gravity. The phenomenon of leaching and the concept of leaching requirement can be better described through a general and fundamental salt balance relationship. Such a relationship, containing all the possible input and output components has been described by Rhoades (1974). Since many of the components considered by him may have insignificant contribution to the overall salt balance, a simpler salt balance model is sufficient to understand the leaching requirement. The simple salt balance model for irrigated lands can be written in words as:
Mass of Salt Added to the Soil = Mass of Salt Extracted from the Soil + Change in the Salt Content of the Soil.
The above statement can be mathematically expressed as Eqn. (13.1) of Lesson 13. The salt addition to the soil is through irrigation water and through the capillary rise of groundwater. The salt extraction takes place through the water that deep percolates beyond the root zone.
For using Eqn. (13.3) of Lesson 13, let’s consider that the irrigation requirement for a three-month cropping season is 275 mm and the electrical conductivities of irrigation water and deep percolated water are 1 and 6 dS/m, respectively (Bhattacharya and Michael, 2003). Therefore, leaching requirement (LR) from Eqn. (13.3) will be:

The total water needed to satisfy both irrigation and leaching requirement is 321 mm. Since, irrigation application efficiency is less than 10%, actually more than the above quantity of water is to be applied. For example, if the irrigation application efficiency is 75%, the total amount of water to be applied to satisfy crop water requirement is 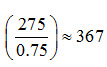 mm in three months, which is more than the sum of the net irrigation requirement (275 mm) and the leaching requirement (46 mm). The excess water application due to lower than 100% application efficiency will eventually deep percolate beyond the root zone. Therefore, the application of a total of 367 mm water in three months will take care of both the irrigation and leaching requirements.
mm in three months, which is more than the sum of the net irrigation requirement (275 mm) and the leaching requirement (46 mm). The excess water application due to lower than 100% application efficiency will eventually deep percolate beyond the root zone. Therefore, the application of a total of 367 mm water in three months will take care of both the irrigation and leaching requirements.
(2) Leaching Efficiency
Recall Eqn. (13.6) which is given as:
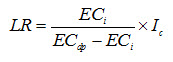
Note that ECdp is the electrical conductivity of the deep percolated water beyond the root zone. This water may be considered as the subsurface drainage effluent, if there is a subsurface drainage system to intercept the water deep percolating beyond the root zone. Gravitational water movement starts when the soil moisture content exceeds the field capacity. Therefore, the electrical conductivity (EC) of the deep percolated water may be roughly assumed to be the same as the electrical conductivity of the soil solution at field capacity (ECfc), i.e., ECdp = ECfc. This will be the case when all of the deep percolated water actually travels through the soil pores before leaving the root zone. However, the water added at the soil surface for the leaching of salts does not move in such a fashion. Normally, a fraction of the added water will deep percolate beyond the root zone faster without mixing with the soil water and the remaining fraction will mix with the soil water and the mixture will move down. If Le is the fraction of added water that moves down after mixing, then (1-Le) is the fraction that moves without mixing. Considering that the added water is the irrigation water (I) with electrical conductivity of ECi and ECdp is the overall electrical conductivity of the deep percolated water (including both the above-mentioned fractions), then more correct expression for ECdp will be:
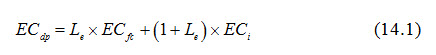
Substituting the above value of ECdp in Eqn. (13.6) of Lesson 13, we have:
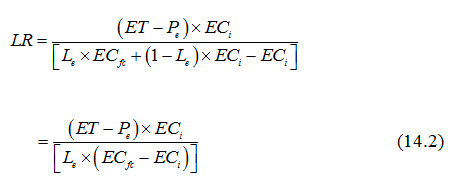
The term Le in Eqn. (14.2) is known as ‘leaching efficiency’ or ‘leaching efficiency fraction’ or ‘leaching efficiency factor’ and its theoretical limits vary from 0 to 1. In using Eqn. (14.2), a maximum permissible value of ECfc is to be assigned. Also, Eqn. (14.2) is valid for the dry season when ET exceeds effective precipitation (Pe). In the wet season, the rainfall is assumed to take care of leaching. The EC of the irrigation should be known. The total water to be applied is the sum of the irrigation requirement and the leaching requirement. For the low-level of salinity hazard, it may not be necessary to estimate leaching requirement separately as the efficiency of water application in the surface methods of irrigation (e.g., border, check basin, and furrow) is generally low. This means that more water is invariably applied on the cropped land than is utilized by the plants. This excess water application satisfies the leaching requirement. The leaching efficiency is low (0.2 to 0.5) for fine-textured soils and is relatively high (>0.5) for coarse-textured soils. The leaching operation becomes more efficient, if the calculated leaching requirement is given in split applications rather than dumping the entire water on the soil surface at one time (Bhattacharya and Michael, 2003). For an effective leaching, the field should be bunded in small units and levelled such that there is no runoff loss and the water spread is of uniform depth on each land unit to be leached.
To demonstrate the use of Eqn. (14.2), let the ET for a cropping season of 3 months is 300 mm, the effective rainfall during this period is 25 mm, the EC of the irrigation water is 1 dS/m, the maximum permissible average electrical conductivity of the deep percolated water (ECfc) is 6 dS/m and the leaching efficiency is 0.4. Substituting these values in Eqn. (14.2) yields:

If the above calculation pertains to a wheat field, the net irrigation requirement is the difference between ET and Pe which, in this case is 300-25 = 275 mm in 90 days. If the irrigation application efficiency is 75%, the gross amount of water needed at the field head to satisfy the crop water requirement and the leaching requirement is 275+137.5 = 412.5 mm in 90 days » 4.6 mm/day. Since this is greater than the gross irrigation requirement of 367 mm, and gross irrigation requirement comprises the extra water that will eventually percolate beyond the root zone, a total water application of 412.5 mm will be enough. If the irrigation interval is 15 days, the amount of water to be applied at each irrigation (to satisfy both the irrigation requirement and leaching requirement) is » 69 mm. Wheat in many regions of India is given 4 to 5 irrigations. If at the location under consideration the number of irrigations is less than 6, the balance water between the actual number of irrigations and 6 irrigations is to be distributed among the actual number of irrigations to meet the leaching requirement.
An important point that emerges is that the excess water application during irrigation (due to less than 100% irrigation application efficiency) has a beneficial effect in terms of leaching of salts. This benefit will exist as long as there is a provision of evacuating the deep percolated water by a suitable drainage system. Otherwise, the deep percolated water will gradually accumulate over years of irrigation and will result into a shallow water table situation with poor-quality soil water, which will be instrumental in creating salinity hazard in the root zone due to capillary rise (Bhattacharya and Michael, 2003).
(3) Field Estimation
The various assumptions made for the theoretical development of the concept of leaching and the estimation of leaching requirement limit the applicability of the result as a guideline only (Bhattacharya and Michael, 2003). Better estimates of leaching requirement, or the consequence of leaching by different amounts and qualities of the water on the soil salinity reduction, are obtained by field experiment in subsurface drained saline lands. Experiments are conducted by actually undertaking leaching operation and monitoring the changes in the drainage effluent salinity or the average root zone salinity over the period of leaching. The leaching curve, with the ratio of final and initial electrical conductivities of the saturation extract of the root zone soil on the Y axis and the amount of leaching water applied (in depth unit) on the X axis, is a declining curve similar to the infiltration rate curve. As the amount of leaching water increases, the above-mentioned electrical conductivity ratio decreases, assuming an almost constant value beyond a certain depth of applied leaching water. Such curves can be developed for different soil depths to be reclaimed (i.e., excess salts removed). When conducted as a pilot study, the result is applicable over the larger project area having similar soil salinity feature. Although this method gives a reliable result, it is time consuming and requires a subsurface drainage facility in the pilot experimental area (Bhattacharya and Michael, 2003). Also, the applicability of the results of such pilot experiments remain restricted to the area which is represented by the pilot experimental area. Nevertheless, the results are much superior to those obtained by the theoretical estimation procedures. Another field method for estimating leaching requirement is ‘infiltration test’, which is less expensive and less time consuming. The leaching requirement obtained by infiltration tests and that obtained by leaching trials in pilot experimental plots having subsurface drainage facility have been found be in agreement (Dieleman, 1972). Details about infiltration tests for estimating leaching requirement can be found in Bhattacharya and Michael (2003).
14.2.3 Final Remarks
Leaching is an effective process for removing salts from the root zone. In coarse-textured soils and when the water table is deep, the chance of the deeper and saline groundwater rising up and resalinizing the root zone is less. In this situation, the reclamation effect due to leaching may last for several years, provided there is no water table rise. In the shallow water table region and in fine-textured soils in which the leaching of salt solutions to greater depths may be very slow, subsurface drainage at an appropriate depth facilitates the removal of the salt-concentrated water that is leaching downward. This may help in permanent reclamation of the topsoil. However, due to prolonged dry season in monsoon climatic regions (e.g., India) and since leaching is seldom complete, the chance of resalinization of the root zone exists due to upward capillary flux of saline groundwater, if the drainage system operation is discontinued. Discontinuity of the subsurface drainage system operation occurs when the drainage effluent collection sump is not evacuated by continuing pumping operation in a pumped subsurface drainage scheme. Discontinuity may also occur in a gravity subsurface drainage scheme if the subsurface drain outlets and the open outlet drains are blocked because of inadequate maintenance. This possibility of resalinization of the root zone under the above situations is strong (Bhattacharya and Michael, 2003).
14.3 Reclamation of Alkali Soils
14.3.1 Introduction
In alkali and acid soils, chemical amendments are required for neutralizing alkali and acid first. Subsurface drainage removes the reaction products of neutralization and restores the soil health. In the case of alkali soils, since the alkali hazard is mostly due to the presence of excess of Sodium, the additive chemical should be able to replace the absorbed Sodium from the soil surface. Gypsum (hydrated Calcium Sulphate) is the most widely used chemical additive for the replacement of Sodium.
14.3.2 Gypsum Requirement for Reclaiming Alkali Soils
Gypsum (powdered hydrated Calcium Sulphate - CaSO4, 2H2O) is a commonly used chemical amendment to the alkali soil for replacing the absorbed Sodium by Calcium. The replaced Sodium, in the form of aqueous solution of Sodium Sulphate, is to be removed from the soil profile through drainage so that it may not again get attached to the soil when Calcium is eventually depleted and the Sodium ion concentration becomes more than the Calcium ion concentration in the soil. The amount of gypsum to be added to the alkali soil for its reclamation is a function of the amount of exchangeable Sodium or more correctly, it depends upon how much of the exchangeable Sodium is to be replaced by Calcium. It also depends upon how much mass of the alkali soil is to be reclaimed (i.e., subjected to replacement of Sodium by Calcium). The calculation of gypsum requirement is explained below through an example.
In the 0-60 cm soil profile at a location in the village Olak, in Taluka Lakhtar of Surendranager district in Gujarat, the average exchangeable cations are as follows (Yadav, 1981):
Ca2+: 7.5 meq/100 g, Mg2+: 6.6 meq/100 g, Na+: 12.3 meq/100 g, and K+: 1.2 meq/100 g.
Assuming that the other exchangeable cations are present in negligible quantities, the cation exchange capacity (CES) is the sum of the above, i.e., 27.6 meq/100 g. The exchangeable Sodium percentage (ESP) is 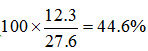 . According to the classification of salt-affected soils described in Section14.1, this soil is highly sodic. If the ESP is to be reduced to below 15%, one has to replace enough Sodium by Calcium (of gypsum) such that the exchangeable Sodium comes down to about 4 meq/100 g; note that
. According to the classification of salt-affected soils described in Section14.1, this soil is highly sodic. If the ESP is to be reduced to below 15%, one has to replace enough Sodium by Calcium (of gypsum) such that the exchangeable Sodium comes down to about 4 meq/100 g; note that ![]() . Thus, Sodium to the extent of (12.3-4) = 8.3 meq/100 g of soil is to be replaced by Calcium. In other words, the Calcium application should be at the rate of 8.3 meq/100 g of soil.
. Thus, Sodium to the extent of (12.3-4) = 8.3 meq/100 g of soil is to be replaced by Calcium. In other words, the Calcium application should be at the rate of 8.3 meq/100 g of soil.
The equivalent weight of Calcium Sulphate (CaSO4, 2H2O) is its molecular weight divided by the valency of the basic element present in it. Hence, one equivalent weight of CaSO4, 2H2O contains 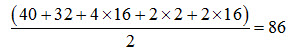 g of Calcium Sulphate. The equivalent
g of Calcium Sulphate. The equivalent
weight of Calcium in gypsum is its atomic weight divided by the valency 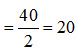 g. Thus, 86 g of gypsum contains 20 g of calcium.
g. Thus, 86 g of gypsum contains 20 g of calcium.
The amount of Calcium required in milligramme is the amount in milliequivalent times the equivalent weight. Hence, mg of Calcium required is 8.3 ´ 20 = 166 mg/100 g of soil. The corresponding amount of gypsum required is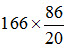 = 713.8 mg/100 g of soil.
= 713.8 mg/100 g of soil.
In the usual process of reclamation of alkali soils through the application of gypsum, a small topsoil layer of 15 to 30 cm depth is considered for reclamation. It is presumed that once this top layer is reclaimed, it will promote good seed germination and enough plant growth. Reclamation of deeper layers by further application of amendment may be taken up later if there is a need felt to do so (Bhattacharya and Michael, 2003). Therefore, in this example, we shall find the gypsum requirement to reduce ESP from the exiting value of 44.6 to 14.5% in top 15 cm of the soil. Assuming an average dry bulk density of the top 15 cm soil as 1.3 g/cm3 (or, 1300 kg/m3), the mass of soil in 15 cm depth of 1 ha land is 19,50,000 kg or 1950 tonne (t). Earlier, we have found that gypsum is to be applied at the rate of 713.8 mg/100 g of soil which equals to 0.007138 kg of gypsum per kg of soil. Hence, the gypsum requirement for reclaiming the top 15 cm soil in 1 ha area is: 0.007138 ´ 1950000 kg = 13919.1 kg » 13.9 t/ha.
Note that the actual gypsum requirement will be more than the above calculated value due to three reasons (Bhattacharya and Michael, 2003). Firstly, during reclamation, one-to-one replacement of Sodium by Calcium does not take place. Secondly, the whole of gypsum may not come in solution if the amount of water added is not adequate. The average value of solubility of gypsum is 0.25% (i.e., 0.0025 kg in 1 liter of water). Addition of such a high quantity of water in one application will invariably cause percolation loss of water without fully dissolving some of the applied gypsum. Thirdly, the commercially available gypsum is not 100% pure.
For the first reason, it is recommended that the actual gypsum application may be 1.25 times the value calculated above (USDA, 1954). To take care of the second reason, gypsum should be applied in split doses with lower quantity of water applied each time. To compensate for the impurity, the result of gypsum calculation should be divided by the purity expressed in fraction.
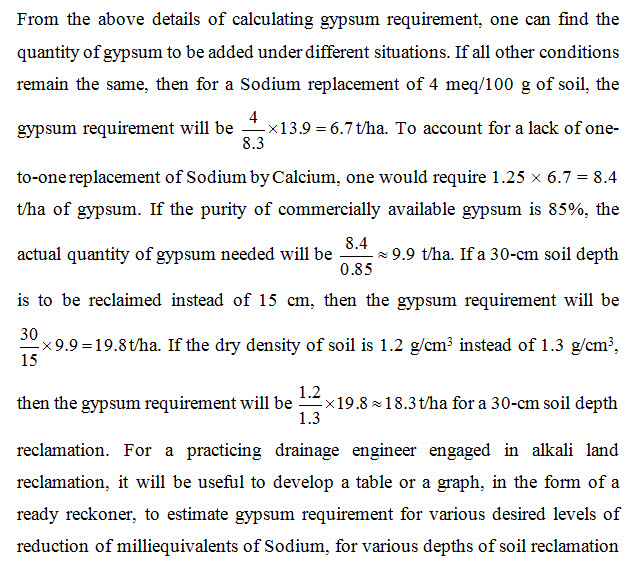
and for various dry densities of soil. The value obtained from the ready reckoner can be suitably adjusted for the lack of one-to-one correspondence between Calcium applied and Sodium removed and for the purity level of the available gypsum. A condensed sample table of gypsum requirement for selected values of milliequivalents of Sodium replacement and for three dry densities of field soil is shown as Table 14.2.
Table 14.2. Gypsum requirement using pure gypsum, one-to-one replacement of Sodium by Calcium and complete dissolution and mixing of gypsum (Source: Bhattacharya and Michael, 2003)
|
Sodium Replacement (meq/100 g of Soil) |
Gypsum Requirement (t/ha) for Reclaiming 15 cm Field Soil at Different Dry Densities |
||
|
1.2 g/cm3 |
1.4 g/cm3 |
1.6 g/cm3 |
|
|
2 |
3.1 |
3.6 |
4.1 |
|
4 |
6.2 |
7.2 |
8.3 |
|
6 |
9.3 |
10.9 |
12.4 |
|
8 |
12.4 |
14.5 |
16.5 |
|
10 |
15.5 |
18.1 |
20.7 |
Note: All values are rounded off to one place after decimal. For field application, multiply table values by 1.25 and divide by the purity of gypsum in fraction. To estimate Gypsum requirement for other values of Sodium replacement, soil depth and average bulk density, use linear interpolation/extrapolation.
For getting best results from the field application of gypsum, the soluble salts should first be leached out of the soil as much as practicable. This enhances the efficiency of utilization of the Calcium present in gypsum for replacing the adsorbed Sodium in the soil (Bhattacharya and Michael, 2003). However, this step may not be feasible to follow if the soil particles are so dispersed in an alkali soil of fine texture that the soil pores are blocked to permit water movement and leaching. Gypsum in a powdered form is usually broadcast on the field and then mixed with the top soil layer using a suitable implement (e.g., a disc plough). After broadcast and mixing, adequate water is added to the field to dissolve the gypsum. The replacement reaction of Sodium by Calcium takes place in the solution phase. It is highly desirable to facilitate leaching out the reaction product in solution from the soil through a subsurface drainage system to get rid of the harmful Sodium.
14.3.3 Final Remarks
Gypsum is one of the several chemical amendments that can be applied for alkali land reclamation. Some of the other chemical amendments are: Sulphur, Sulphuric acid (H2SO4), Iron Sulphate (FeSO4, 7H2O), Calcium Chloride (CaCl2), Calcium Carbonate (CaCO3) and Aluminium Sulphate {Al2(SO4)3, 18H2O}. The quantities of the above chemical amendments equivalent to 1 unit of gypsum is obtained by multiplying the gypsum quantity with the ratio of the equivalent weights of the amendment and the equivalent weight of gypsum. Accordingly, the following equivalent quantities are obtained for different amendments corresponding to 1 unit of gypsum (Bhattacharya and Michael, 2003):
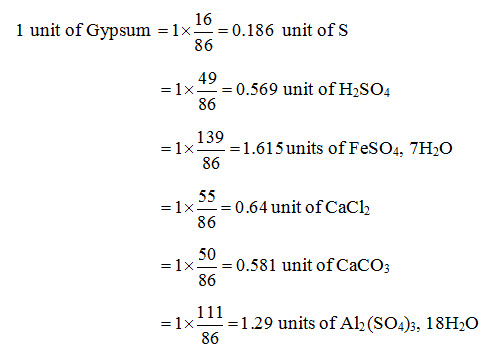
The unit in all the cases is the same, for example, tonnes per hectare (t/ha).
It should be noted that in the amendments which do not contain Calcium, the reaction with water first produces Sulphuric acid which then reacts with available Calcium Carbonate in the soil to produce Calcium Sulphate, the Calcium of which replaces the Sodium. In India, gypsum is preferred and is the most commonly used chemical amendment for alkali land reclamation because of its easy availability and low cost (Bhattacharya and Michael, 2003).
14.4 Reclamation of Acid Soils
14.4.1 Lime Requirement for Reclaiming Acid Soils
Lime is the most commonly used chemical additive in acid soils to neutralize the soil acidity. As gypsum requirement for the reclamation of alkali soil is determined on the basis of desired replacement of Sodium from the soil complex, in principle, the lime requirement is determined based on the requirement of raising soil pH from less than 6 to a desirable higher value (Bhattacharya and Michael, 2003). Theoretically, the lime requirement depends on the extent by which the exchangeable H is to be replaced by the Calcium present in the lime. Calculation of lime requirement through pH determination of the soil has several procedures and they give widely varying results (Peech, 1965). The one that is recommended in India (Singh et al., 1999) is based on the procedure suggested by Shoemaker et al. (1961). In this procedure, a known mass of soil is treated with a buffer solution of known pH and the resulting pH of the suspension is measured with a pH Meter after standardizing it with buffer solutions of known pH. The lime requirement for the original soil (from which the sample was taken) is read from a ready reckoner table, which gives the lime requirement of the soil to raise its pH from the known lower value to a desired higher value. For example, if the pH of the soil sample prepared in the above fashion is 5.0 and it is to be raised to 6.0, the theoretical lime requirement will be close to 20 t/ha. The actual lime requirement is determined by dividing the theoretical requirement by the purity of lime (in fraction). The calculated lime quantity is for increasing the soil pH of the plough layer, which is generally considered as 15 cm.
From the above discussion, it is apparent that the calculated lime requirement is huge, particularly if a greater depth of soil is considered for raising pH or if the pH is to be raised by a greater extent. However, in practice much lesser lime application rate has been found to achieve the goal of increasing agricultural production in acid soils. The phenomenon responsible for this is not well understood (Bhattacharya and Michael, 2003). For instance, Panda et al. (1996) reported that by reducing lime application from full dose once at the beginning of a six-year field experiment to one-twentieth of the full dose at the sowing time every year, the reduction in yield of green gram, groundnut, black gram, soybean, lentil and pea varied between 8 and 11%. With lime application every year at the time of sowing at one-fifteenth of the lime requirement, the yield reduction of the above crops were less than 8% as compared to when the lime was applied at full dose once at the beginning of the six-year experiment. In the acid sulphate soils of Kerala, the farmers’ conventional practice is to apply about 600 kg of lime (CaO) at the time of preparation of rice fields every year. There are several other sources in the literature, which also show that the lime applied at much lesser than the calculated dose considerably increases the production of various crops in acid soils.
14.5 Concluding Remarks
In a nutshell, for the reclamation of chemically degraded soils, usually one has to determine the leaching requirement for saline soils, gypsum requirement for alkali soils and the lime requirement for acid soils. In general, salinity can be controlled by natural leaching if the water table is deep, salt washing from the land surface by runoff or by additional good-quality water, or by subsurface drainage. Control of alkali and acidity is best achieved by adopting drainage and using suitable chemical amendments. Under alkali conditions, particularly in fine-textured soils, the application of chemical amendment is necessary to improve the water transmission characteristics through the soil. Simultaneous adoption of subsurface drainage removes the reaction products from the soil system. Due to arid and semi-arid climate over several parts of India, there are chances of resalinization and reconversion to alkali condition. Therefore, subsurface drainage operation should not be discontinued even the soil has been fully reclaimed (Bhattacharya and Michael, 2003).
References
Abrol, I.P. and Gupta. R.K. (1991). Salt Affected Soils, Their Diagnosis and Management. National Training Course on Field Drainage for the Control of Groundwater Table and Soil Salinity in Irrigated Lands of Semi-Arid Regions, Central Soil Salinity Research Institute (CSSRI), Karnal, Haryana, pp. 1-37.
Anonymous (1976). Acid Soils of India: Their Genesis, Characteristics and Management. Bulletin No. 11, Indian Society of Soil Science, Division of Soil Science and Agricultural Chemistry, IARI, New Delhi.
Bhattacharya, A.K. and Michael, A.M. (2003). Land Drainage: Principles, Methods and Applications. Konark Publishers Pvt. Ltd., New Delhi.
Dieleman, P.J. (Editor) (1972). Reclamation of Salt Affected Soils in Iraq. ILRI Publication No. 11, International Institute of Land Reclamation and Improvement (ILRI), Wageningen, The Netherlands.
Mathew, E.K., Nair, M. and Raju, T.D. (1993). Drainage and Rice Productivity. Technical Bulletin, AICRP on Agricultural Drainage, Kerala Agricultural University, Karumady, Kerala.
Panda, N., Sarkar, A.K. and Mathur, B.S. (1996). Chemical Leading to Soil Acidity and its Amelioration. In: T.D. Biswas and G. Narayansamy (Editors), Soil Management in Relation to Land Degradation and Environment, Bulletin No. 17, Indian Society of Soil Science, Division of Soil Science and Agricultural Chemistry, IARI, New Delhi, pp. 82-88.
Peech, M. (1965). Lime Requirement. In: C.A. Black (Editor-in-Chief), Methods of Soil Analysis Part 2, Monograph No. 9, American Society of Agronomy, Madison, Wisconsin.
Rehman, F. (1991). Maximization of Tea yields. In: The Seed that Bore Fruit, Centenary Volume of Mornai Tea Estate, Publ. Northern Evangelical Lutheran Church, Dumka, Bihar.
Rhoades, J.D. (1974). Drainage for Salinity Control. In: Jan Van Schilfgaarde (Editor), Drainage for Agriculture, Monograph No. 17, American Society of Agronomy, Madison, Wisconsin.
Shoemaker, H.E., McLean, E.O. and Pratt, P.F. (1961). Buffer methods for determining lime requirement of soils with appreciable amounts of extractable aluminium. Proc. Soil Sci. Soc. of America, 25: 274-77.
Singh, D., Chhonkar, P.K. and Pandey, R.N. (1999). Soil Plant Water Analysis: A Methods Manual. Indian Agricultural Research Institute, New Delhi.
Singh, N.T. (1994). Land Degradation and Remedial Measures with Reference to Salinity, Alkalinity, Waterlogging and Acidity. In: D.L. Deb (Editor), Natural Resource Management for Sustainable Agriculture and Environment, Angkor Publishers (P) Ltd., New Delhi.
Suraj Bhan (1995). Reclaim your salt-affected soils. Journal of Soil and Water Conservation, 39(3&4): 173-81.
USDA (1954). Diagnosis and Improvement of Saline and Alkali Soils. USDA Handbook No. 60, U.S. Salinity Laboratory, United States Department of Agriculture (USDA), Washington DC.
Yadav, J.S.P. (1981). Research on Salt Affected Soils and Saline Water 1971-1981. Progress Report of Coordinated Project for Research on Management of Salt Affected Soils and Use of Saline Water in Agriculture. Indian Council of Agricultural Research (ICAR), New Delhi.
Suggested Readings
Bhattacharya, A.K. and Michael, A.M. (2003). Land Drainage: Principles, Methods and Applications. Konark Publishers Pvt. Ltd., New Delhi, India.
Murty, V.V.N. and Jha, M.K. (2011). Land and Water Management Engineering. Sixth Edition, Kalyani Publishers, Ludhiana, India.
Ritzema (Editor-in-Chief) (1994). Drainage Principles and Applications. International Institute for Land Reclamation and Improvement (ILRI), ILRI Publication 16, Wageningen, The Netherlands.
Schwab, G.O., Fangmeier, D.D., Elliot, W.J. and Frevert, R.K. (2005). Soil and Water Conservation Engineering. Fourth Edition, John Wiley and Sons (Asia) Pte. Ltd., Singapore.
Smedema, L.K. and Rycroft, D.W. (1983). Land Drainage. Batsford Academic and Education Ltd., London.