Site pages
Current course
Participants
General
Module 1. History and types of greenhouse
Module 2.Function and features of greenhouse
Module 3.Scope and development of greenhouse techn...
Module 4.Location, planning and various components...
Module 5.Design criteria and calculations
Module 6. Construction materials and methods of co...
Module 7. Covering material and characteristics
Module 8. Solar heat transfer
Module 9. Solar fraction for greenhouse
Module 10. Steady state analysis of greenhouse
Module No. 11 Greenhouse Heating, Cooling, Sheddin...
Module 12. Carbon dioxide generation and monitorin...
Module 13. Instrumentation and & computerized ...
Module 14. Watering, fertilization, root substrate...
Module 15. Containers and benches
Module 16. Plant nutrition, Alternative cropping s...
Module 17. Plant tissue culture
Module 18. Chemical growth regulation
Module 19. Disease control, integrated pest manage...
Module 20: Post Production Quality and Handling
Module 21: Cost analysis of greenhouse Production
Module 22. Application of greenhouse & its rep...
Lesson 22 Watering, Fertilization, Root substrate and Pasteurization
22.1 INTRODUCTION
Watering is the greenhouse operation that most frequently accounts for loss in crop quality. When performed correctly it is simple operation otherwise it results either into underwatering or overwatering. A wide variety of inexpensive automatic watering systems are available today.
Fertilizers are designed to provide the elements necessary for plant growth. About 90% of the plant weight is made up of water. The remaining mass constitutes the plant dry weight, which is made up primarily of 17 elements that are required for plant growth.
Root medium pasteurization is a standard practice for virtually all greenhouses today. It is generally done on annual basis, although number of growers are pasteurizing their media every crop. The summer has been a preferred time for pasteurization because crop production is usually at a low point, root media are warmer and in case of steam pasteurization, all or much of boiler capacity is available at this time. Root medium pasteurization, in addition to eliminating disease organisms, is used to control nematodes, insects and weeds. Pasteurization may be accomplished by injecting steam into the soil or by injecting one of several chemicals such as methyl bromide chloropicrin.
22.2 WATERING
1. Judge watering requirements by substrate look, feel, and weight
2. Plant symptoms of underwatering:
Wilting
Slowed growth
Smaller leaves
Possible leaf burn
3. Plant symptoms of overwatering:
Excess growth
Soft growth
Possible root damage
Wilts easily under strong light
22.2.1 WATERING SYSTEMS
Water is primarily supplied to the system through water mains installed underground or overhead. Two-inch PVC pipes are commonly used in 20,000 sq. ft. greenhouses, and 3-inch pipes are used in 50,000 sq. ft. greenhouses. Double water mains may need to be installed for fertilizer application. Water efficiency can be improved with use of pulse watering system, where plants receive maximum water without runoff (i.e., boom watering).
22.2.1.1 Hand watering
Not economical due to labour costs
Beneficial for spot watering
Water supplied through hand-held field hose
Water breaker should be installed on end of hose
22.2.1.2 Perimeter Watering
Plastic polyethylene or PVC pipe run along bench edges
Water is sprayed under foliage through nozzles that are staggered along the pipe
Nozzles can spray 180°, 90°, or 45°
Water is projected farther into bed by 90° and 45° nozzles
Nozzles are attached by holes punched
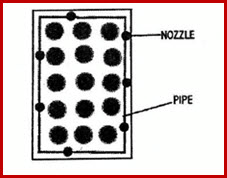
Fig 22.2.1 Perimeter Watering
22.2.1.3 Twin-Wall Watering
Good for long or sloping benches
Constant water pressure along tube
Tube consists of two sections:
- Outer chamber
- Inner chamber
Water first enters the tube in the outer chamber through a special pipe fitting connected to the water supply
Water moves down the length of the tube until it reaches the end, where it begins to enter small pores along the tube leading to the inner chamber
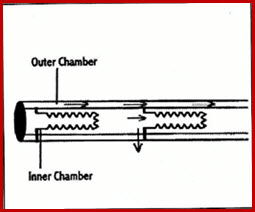
Fig 22.2.2 Twin Wall Watering
22.2.1.4 Tube Watering
Polyethylene micro-tubes run from water supply to each individual pot.
Emitters are attached to the end of the tube.
Water is supplied by ¾-inch polyethylene or PVC pipes run along the centre of the bench
Tubes are attached to the pipe through drilled holes.
Consistent tube length is required.
Benches should be level to insure even watering.
Method can also be used for hanging plants.
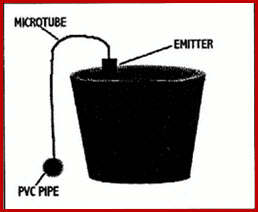
Fig 22.2.3 Tube Watering
22.2.1.5 Overhead Watering
Water is applied through 360° nozzles attached to top of riser pipes
Nozzles may be designed to rotate 360°
Riser pipes are periodically attached to a pipe run along the centre of the bed
Riser pipes reach well above plant tops
22.2.1.6 Boom Watering
Boom runs along rails attached down centre of greenhouse
Boom is propelled by an electric motor
Can be programmed to water one side only or to skip sections of the greenhouse
Good example of pulse watering
22.2.1.7 Mat Watering
Good for several pot sizes
Polyethylene sheets are placed on benches
A 3/16 to ½ inch thick moist mat is placed on top of the sheets
Pots are placed on the mat, then take up water through holes on the bottom through capillary action
Very important that pots have bottom holes
Once pot is lifted from mat, capillary action is broken and it becomes necessary to re-water pot from top to re-establish capillary action
Benches should be level to insure even watering
To prevent algae, perforated polyethylene may be placed on top of mat for pots to sit on
Watering tubes placed 2 feet apart run down the length of the bench to supply water to the mat
22.2.1.8 Ebb-and-Flood Watering System
Pots are placed in a level, watertight bench
The bench has channels in the bottom and a hole in the centre for the water to enter and exit
A filter and a tee valve are installed in the hole
Water is pumped into bench to a level of ¾ to 1 inch over 10 minutes
Pots are allowed to sit in water for 10-15 minutes
Water is drained out over 10 minutes
Easy to change pot sizes
High humidity may cause problems
22.2.1.9 Flood Floor Watering System
Greenhouse floor is paved with a slight slope toward the centre on either side or a lip that runs along the perimeter
A drain hole is installed in the centre
Hot-water heating pipes are installed to speed up the time needed to dry the floor to lower relative humidity
Flood greenhouse with water
Time required to flood greenhouse will vary
22.2.1.10 Trough Watering
Troughs containing one row of plants are placed parallel down the greenhouse with spaces in between
- Reduces humidity
- Promotes dryer foliage
Troughs are slightly sloped for the water to drain into a gutter where it is returned to a holding tank
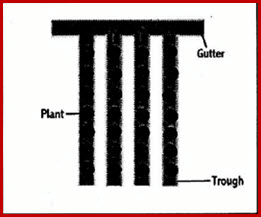
Fig 22.2.4 Trough Watering
22.3 FERTILIZATION
In protected cultivation, the cost of fertilization is small in relation to the vegetable production costs, so fertilization has been usually high, as growers have no incentives to save fertilizers and pretend, mistakenly in many cases, that the crop did not suffer any kind of nutrient deficiency. Nevertheless, nowadays, the trend to minimize the environmental impact has resulted in the adoption of the so called ‘Good agricultural practices (GAP) code’.
22.3.1 The nutrients cycle (soil cultivation)
In horticulture, and especially in greenhouses, there is more leaching of nitrates than in other agricultural systems, due to the high supplies, the high contents of organic matter in the soil and the surplus irrigation in relation to the ETc (Dasberg,1999b). Its environmental impact can be notable, mainly, in the surface and underground aquifers.
The applications of nitrogen fertilizers in the greenhouses normally exceed the crop’s requirements, increasing the risks of nitrate leaching to the aquifers (Thompson et al., 2002).
Phosphorus does not usually cause pollution problems, except in exceptional cases of soils with low phosphorus fixation capacity or when large quantities of animal manure are applied over many years.
The potassium leachate is, normally, limited and does not cause important problems of environmental impact, as it is retained in the soil in high proportions.
Other macronutrients, such as calcium and magnesium, do not cause environmental problems, as they are natural components of the soil, which retains them in large quantities.
22.3.2 Nutrient Extraction
It is necessary to know the fertility characteristics and nutrient levels in the soil, making the pertinent soil analysis, to schedule fertilization. Normally, if the nutrient level is good, fertilization in practice is based on supplying the crop’s uptake, corrected for the use efficiency, which allows for maintaining, after the crop cycle, a proper fertility and nutrient level. If the levels of any nutrient are high, or if the irrigation water contains it in sufficient amount, the inputs must be consequently corrected.
As a guide, Table 22.3.1 summarizes the approximate nutrient uptakes of some horticultural crops.
It is important to know the nutrient absorption dynamics, to adapt the inputs to the extraction rates, which vary through the cycle and are influenced by the climate conditions, especially by soil temperature and radiation.
When the availability in the soil of some nutrients is high, over consumption may occur (in potassium) or it may negatively affect the quality (nitrogen) of the fruits, in extreme cases being possible to induce salinity, or even phytotoxicity. The availability of nutrients must be balanced and adapted to the plant requirements, to avoid antagonisms and possible restrictions to the nutrient absorption, which allow for optimum fertilization. Therefore, it is frequent to maintain predetermined relations between all or some of the nutrients
Table 22.3.1 Approximate nutrient uptake of some horticultural crops (compiled from very diverse sources).
|
Crop |
Yield (t/ha) |
Plant Uptake (Kg/ha) |
||||
|
N |
P2O5 |
K2O |
CaO |
MgO |
||
|
Tomato |
80 |
250 |
80 |
500 |
300 |
70 |
|
Pepper |
40 |
180 |
60 |
180 |
160 |
50 |
|
Aubergine |
50 |
250 |
40 |
300 |
150 |
25 |
|
Melon |
60 |
230 |
80 |
400 |
300 |
70 |
|
Cucumber |
200 |
320 |
160 |
600 |
250 |
100 |
|
Squash |
40 |
70 |
70 |
390 |
- |
- |
|
Lettuce |
40 |
100 |
50 |
250 |
50 |
12 |
|
Green bean |
45 |
150 |
15 |
60 |
30 |
6 |
22.3.3 Tolerance to salinity
The tolerance to salinity of the crops may be assessed in several ways. The most extensively used method (Ayers and Westcot,1976) quantifies the tolerance (Table 22.3.2) by the percentage of the maximum yield that would be obtained for a certain level of electric conductivity of the saturated extract of the soil (ECe) or the irrigation water (ECw) used. For greenhouse crops the tolerance to salinity can be quantified (Sonneveld, 1988) by means of the irrigation water salinity threshold below which there is no problem, and the percentage of yield decrease experienced by the crop per unit increase of salinity in the irrigation water, above the threshold value. This method is more useful for substrate crops. Table 22.3.3 summarizes the data in this respect. The specific growing conditions (cultivar, evaporative demand, management, and microclimate) may affect these threshold values (Cohen, 2003). In Mediterranean greenhouses, Magan (2003) estimated the salinity threshold value of the nutrient solution to decrease the fresh weight tomato harvest between 4 and 5 dS m-1.
The importance of the water quality to minimize the leaching fraction is enormous, influencing its environmental impact potential. Poor quality water will require considerable leaching and, as a consequence, will generate more negative impact than good quality water.
Table 22.3.2 Tolerance level of some crops to salts (dS m−1), expressed as the expected yield (in percentage of the maximum yield).
(Source: Ayers and Westcot, 1976.)
|
Percentage of maximum yield |
|||||||||
|
Crop |
100% |
90% |
80% |
50% |
|
||||
|
ECw |
ECe |
ECw |
ECe |
ECw |
ECe |
ECw |
ECe |
Max ECea |
|
|
Climbing bean |
0.7 |
1.0 |
1.0 |
1.5 |
1.5 |
2.3 |
2.4 |
3.6 |
6.5 |
|
Broccoli |
1.9 |
2.8 |
2.6 |
3.9 |
3.7 |
5.5 |
5.5 |
8.2 |
13.5 |
|
Melon |
1.5 |
2.2 |
2.4 |
3.6 |
3.8 |
5.7 |
6.1 |
9.1 |
16.0 |
|
cucumber |
1.7 |
2.5 |
2.2 |
3.3 |
2.9 |
4.4 |
4.2 |
6.3 |
10.0 |
|
Potato |
1.1 |
1.7 |
1.7 |
2.5 |
2.5 |
3.8 |
3.9 |
5.9 |
10.0 |
|
Lettuce |
0.9 |
1.3 |
1.4 |
2.1 |
2.1 |
3.2 |
3.4 |
5.2 |
9.0 |
|
Onion |
0.8 |
1.2 |
1.2 |
1.8 |
1.8 |
3.2 |
2.9 |
4.3 |
8.0 |
|
Pepper |
1.0 |
1.5 |
1.5 |
2.2 |
2.2 |
3.3 |
3.4 |
5.1 |
8.5 |
|
Spinach |
1.3 |
2.0 |
2.2 |
3.3 |
3.5 |
4.9 |
5.7 |
8.6 |
15.0 |
|
Strawberry |
0.7 |
1.0 |
0.9 |
1.3 |
1.2 |
1.8 |
1.7 |
2.3 |
4.0 |
|
Tomato |
1.7 |
2.5 |
2.3 |
3.5 |
3.4 |
5.0 |
5.0 |
7.6 |
12.5 |
Table 22.3.3 Tolerance of some vegetables to salinity in greenhouses
|
|
Threshold value ECw (ds m-1) |
Yield decrease by salinity (%) |
|
Tomato |
1.8 |
9 |
|
Pepper |
0.5 |
17 |
|
Cucumber |
1.5 |
15 |
|
Green Bean |
0.5 |
20 |
|
Lettuce |
0.6 |
5 |
22.3.4 Fertigation
This practice of joint application of irrigation and fertilization is known as fertigation. The control centre of a localized irrigation facility must have the necessary equipment to fertigate. This involves the use of soluble or liquid fertilizers, allowing for adjustable dosing and fractioning of the inputs which optimizes their use.
22.3.4.1 Criteria of fertigation
Traditionally, the fertigation criterion of supplying the nutrients as a function of the expected uptake by the plants prevailed.
Nowadays, the criterion of providing nutrients based on an ionically balanced physiological solution, used in soilless crops, is extending to conventional soil cultivation, when a suitable automated irrigation head is available.
In soilless cultivation the correction of the nutrient solution is performed based on its analysis. In soil cultivation, the classic method of analysing the saturated soil extract is being replaced by the use of suction probes, with which a sample of the soil solution is extracted for analysis. However, information on the ideal nutrient levels to use with this method is still scarce.
22.3.4.2 A practical example: a soil-grown tomato crop
Depending on each case’s specific conditions (soil fertility, climate and irrigation type), there is notable variation in tomato fertilization (Castilla, 1995). Preliminary analysis of the soil is necessary. In general, fertilizers are applied depending on the crop’s estimated nutrient uptake. Although the variability in nutrient uptake is enormous, values that refer to harvest unit are in general lower.
Between 2.1 and 3.8 kg of N t-1 of harvest;
Between 0.3 and 0.7 kg of P t-1 of harvest;
Between 4.4 and 7.0 kg of K t-1 of harvest;
Between 1.2 and 3.2 kg of Ca t-1 of harvest; and
Between 0.3 and 1.1 kg of Mg t-1of harvest.
The differences in nutrient uptakes are influenced by the type of pruning and, especially, by the timing of the removal of the axillary shoot. It is advisable to prune shoots as soon as possible to minimize the wasteful uptake of nutrients by the crop.
The scheduling of fertilizer application must rely on the type of fertilizer used, on the irrigation technique and on the soil conditions, among other factors. In sandy soils, with low water storage capacity, supplies must be frequent with the irrigation (conventional), whereas in heavy soils it is only necessary to apply part of the nitrogen as a top dressing (Geisenberg and Stewart, 1986).
With surface irrigation, the most common practice is to apply the phosphorus with the pre-planting fertilization, for example when applying manure (around 30 t ha-1), and at a time when half of the potassium is applied. The rest of the potassium and nitrogen are applied in alternate weeks after transplanting until 1 month before the end of the cycle (Nisen et al., 1988). With drip irrigation, all fertilizers can be applied by fertigation, although it is common that at least part of the phosphorus is applied with the manure.
In drip irrigation, it is essential to know the absorption rhythm of the mineral elements in order to schedule fertilization (Zuang, 1982). In Mediterranean unheated greenhouse crops for autumn-spring cycles, fertilization rates higher than 0.3 g N m-2 day-1 do not seem advisable (Castilla, 1985). The fertilizer’s content of the irrigation water is, in some cases, notable and must be taken into account for the fertilization schedule.
Nitrogen excesses negatively affect fruit quality, and maintaining an N:K ratio at 1:2 (or even 1:3) during the fruit enlargement stage, with drip irrigation, favours their quality (Geisenberg and Stewart, 1986). Equally, the balance between other nutrients, especially between calcium (when its supply is required) and potassium, and magnesium is necessary, as well as between the different forms of nitrogen (nitric/ammoniacal).
In drip irrigation, the amount of salts in the water must be limited, if possible, to 2 g l-1 (which is not feasible, in some cases, when saline water is used), to decrease possible dripper blockage problems. When using good quality water, it is a usual practice to add sodium chloride (common salt) to the water, up to the indicated limit, to improve tomato quality, because the soluble solids content increases with salinity which contributes to the improvement of its internal quality, although the fruit size is reduced.
A good irrigation efficiency is, logically, required for efficient fertigation and also contributes to a significant reduction in the environmental impact of fertilizer (nitrogen, especially) residues.
Foliar fertilization, in tomato crops, is usually limited to microelements, when deficiencies are forecasted or observed. Leaf analysis (of the limb, petiole or the whole leaf) is a good auxiliary index on which to base the scheduling of fertilization, being more common than sap analysis, as the latter displays a wider variability and requires more thorough sampling (Chapman, 1973; Van Eysinga and Snilde, 1981; Morard, 1984).
In greenhouse crops, low soil temperatures (15°C) in winter may limit absorption of nutrients, especially phosphorus (Wittwer, 1969) and nitrates (Cornillon, 1977). On the other hand, high temperatures favour nutrient absorption, although the nutrient uptake per harvest unit is not affected, as previously thought (Nisen et al., 1988).
22.3.4.3 Fertigation of soilless crops
22.3.4.3.1 The nutrient cycle in soilless crops
Soilless crops, with free drainage, have similar problems to soil cultivation regarding the nutrient cycles. The management conditions (leaching percentage, characteristics of the nutrient solution) of the soilless crop (open system) will determine its environmental impact, which will be similar to that of crops grown in soil if the leachates are similar. If leachates are recirculated (closed system) the salinity and pathology problems must be considered and the nutrient con centrations must be well monitored and controlled.
22.3.4.3.2 Preparation of the nutrient solution
In an ideal soilless growing system there are no mineral inputs from the substrate, and therefore, all nutrients must be supplied together with the water, in the nutrient solution. The preparation of the nutrient solution requires prior analysis of the irrigation water, to allow for the formulation of the best nutrient solution depending On the crop to be grown. The preparation of this nutrient solution will also depend on the technical characteristics of the available fertigation hardware (and, possibly, software).In the simplest case, one concentrated solution tank is available and another tank for the acid. Most facilities have two tanks for solutions (A and B), one of which already contains the acid. In this case, tank A has most of the acid to correct the pH (usually, nitric or phosphoric, and rarely sulphuric), the phosphates and the sulphates, as well as the microelements, except for iron. In this tank A, part of the potassium nitrate can be incorporated, but no calcium salts must be added, to avoid precipitates. Tank B contains the calcium nitrate and the potassium nitrate (all or only a part), as well as some nitric acid to regulate the pH and the iron chelates. The magnesium nitrate is usually added in tank B, but neither sulphates nor phosphates must ever be added, to avoid precipitates.
When three tanks are available, one of them is destined only for the acid that is usually nitrous acid, although sulphuric or phosphoric acids can be used. In sophisticated facilities, managed by means of a computer, several tanks are usually available, containing solutions of individual fertilizers.
The injection systems of concentrated solutions in the irrigation water flux are of such complexity or simplicity in agreement with the type of tanks used.
In mixing the fertilizers their solubility and compatibility must be taken into account, not forgetting that it depends on temperature (Tables 22.3.4 and 22.3.5). The literature on the preparation of simple solutions is extensive (e.g. Cadahía, 1998).
Table 22.3.4 Solid fertilizers most commonly used in fertigation: analysis and solubility at 20°C.
|
Fertilizer |
Analysis of N-P2O5-K2O-othersa (%) |
Solubility (gl−1)
|
|
Calcium nitrate 4H2O Ammonium nitrate |
15.5-0-0-26.6 (CaO) 33.5-0-0 |
1200 1700b |
|
Ammonium sulfate |
21-0-0-22 (S) |
500 |
|
Urea |
46-0-0 |
500 |
|
Potassium nitrate |
13-0-46 |
100–150 |
|
Potassium sulfate |
0-0-50-18 (S) |
110 |
|
Mono potassium phosphate |
0-52-33 |
200 |
|
Mono ammonium phosphate |
12-60-0 |
200 |
|
Magnesium sulfate 7H2O |
16 (MgO)-13 (S) |
700 |
|
Urea phosphate |
17-44-0 |
150 |
|
Magnesium nitrate 6H2O |
11-0-0-9.5 (Mg) |
500 |
The first three values in each entry refer to N-P2O5-K2O. Where there is a fourth entry this refers to other compounds. The exception is the entry for magnesium sulphate which has no N-P2O5-K2O and contains16 (MgO)-13 (S) as indicated. b Steep water temperature decrease for concentrations above 250 g l−1.
Table 22.3.5 Chemical compatibility of the mixture of some common fertilizers in fertigation: I, incompatible; C, compatible. (Source: Cadahía, 1998.)
|
|
NO3NH4 |
Urea |
(NH4)2SO4 |
(NH4)2HPO4 |
(NH4)H2PO4 |
KCL |
K2SO4 |
KNO3 |
(CaNo3)2 |
|
NO3NH4 |
- |
|
|
|
|
|
|
|
|
|
Urea |
C |
- |
|
|
|
|
|
|
|
|
(NH4)2SO4 |
C |
C |
- |
|
|
|
|
|
|
|
(NH4)2HPO4 |
C |
C |
C |
- |
|
|
|
|
|
|
(NH4)H2PO4 |
C |
C |
C |
C |
- |
|
|
|
|
|
KCL |
C |
C |
C |
C |
C |
- |
|
|
|
|
K2SO4 |
C |
C |
C |
C |
C |
C |
- |
|
|
|
KNO3 |
C |
C |
C |
C |
C |
C |
C |
- |
|
|
(CaNo3)2 |
C |
C |
C |
C |
C |
C |
C |
C |
- |
22.3.4.3.3Parameters of fertigation with soilless crops
The proper management of fertigation requires the periodic analysis of the nutrient solution to assess its goodness of fit to the requirements of the crop and to perform necessary adjustments to its composition. In addition, it is necessary to frequently monitor (automated or manual) the pH and EC (electrical conductivity) of the nutrient solution and the leachate, to prevent any anomaly.
In practice, when a computer is available, a certain threshold of EC of the nutrient solution is fixed, for instance 2.5 dS m-1, modulated as a function of the solar radiation, decreasing it by 0.1 dS m-1 for each 30 W m-2 of solar radiation that exceeds 400 Wm-2 (Urban, 1997b). Obviously, these rules must adapt to the specific conditions of each operation.
Normally, the pH is not regulated but a certain value is fixed. The EC and pH sensors must be duplicated, at least, to prevent an eventual failure. In addition, the system must be fitted with alarms. Other complementary analyses are carried out on the substrate solution (extracted with a syringe) and the drainage, to correct the nutrient solution.
Analysis of vegetable tissue and sap provide information on the nutrients that are really absorbed by the plants (Cadahia, 1998). The analysis of the conducting tissues is usually preferable to that of the leaves, whose composition varies slowly. In these analyses, the time variations are more relevant than the absolute values (Morard et al., 1991).
22.3.4.3.4 Pathogens in the drainage waters
The recirculation of the drainage water requires the use of good quality water and its disinfection, to suppress pathogens (bacteria, fungi and virus) in the recirculation water.
The use of ozone, UV sterilization, thermal treatment and ultrafiltration are effective to a varying extent, but the last technique has the drawback that only 70–80% of the drainage water is recovered (Dasberg, 1999b). The use of bleach in recirculation water dis infection gives good results. Treatment with UV radiation is effective, but it is necessary to pre-filter the water so the radiation penetrates well (Dasberg, 1999b).
22.3.4.3.5 Automation
The use of computers, with several degrees of automation to manage the fertigation, is growing among greenhouse growers. It must be expected, in the future, that these systems will become integrated with climate control systems, in those greenhouses in which the technological level allows for it, for combined optimization.
22.4 ROOT SUBSTRATE
22.4.1 Functions of substrates
Serves as a reservoir for plant nutrients
Serves as a reservoir for water available for plants
Must provide gas exchange between roots and the atmosphere outside the root substrate
Provides anchorage or support for the plant
22.4.2 Limitations of materials
Sand : excellent support and excellent gas exchange but poor water and nutrient-holding capacity
Clay: high nutrient- and water-holding capacity plus excellent support but poor gas exchange
Water : water and nutrients; can even supply gas exchange, but offers no support; if plants are given support
Field soils (when placed in a pot) : excellent support, nutrient-holding capacity, and water-holding capacity, but poor gas exchange
22.4.3 Desirable properties of a substrate
Stability of organic matter
decomposition of organic components = smaller particle size = finer texture= smaller pores = reduced gas exchange and reduced aeration also means a loss of substrate volume
straw and saw dust (excluding some like redwood) are examples of materials with poor stability
Carbon-to-nitrogen ratio
Organic materials are broken down by microbes
Microbes require N for decomposition
If C : N ratio > 30 C : 1 N, & substrate contains
If organic materials decomposes rapidly, the microbes will utilize N
The C : N of sawdust is about 1000 : 1
Pine bark has a C : N of about 300 : 1, yet is still suitable to use…
Dry bulk density
oven-dry weight of substrate particles ÷ volume given in lb/ft3
useful for predicting materials handling
if too low, as the substrate dries out, top-heavy pots topple over
Wet bulk density
weight of substrate at container capacity ÷ volume reported in lb/ft3
container capacity is moisture content of substrate just after complete saturation and loss of gravitational water
([volume needed to saturate - drainage volume] ÷ total volume of container) × 100
usually reported as % of total volume
Moisture retention and aeration
goal is a substrate with adequate available water +sufficient aeration + acceptable wet and dry bulk densities
substrate at container capacity composed of : solid particles
pores filled with : unavailable water & available water (micropores), air (macropores)
Unavailable water (hygroscopic water)
held by solid particles so “tightly” that it is unavailable to roots common.
roots would have to create a suction > 15 bars to separate water from the particles
Available water
volume of water at container capacity – volume of water remaining at 15 bars pressure
Cation exchange capacity
many substrate components have fixed negative electrical charges
will attract and hold positive-charged cations
CEC = milliequivalents per 100 cc of dry substrate
6 to 15 me/100 cc is desirable
clay, peat moss, and coir have higher CEC’s
pH
most greenhouse crops = 6.2 to 6.8 in soil-based substrates (20% or more soil) and 5.4 to 6.0 in soilless substrates
22.5 ROOT MEDIA PASTEURIZATION
Sterilization is the killing of all living organisms on or in a material. Pasteurization is the killing of most living organisms on or in a substance. In the past, the general dogma is that pasteurization kills the harmful organisms (i.e. disease-causing fungi) in a substrate but allows most of the beneficial organisms to live. However, pasteurization does kill many of the beneficial organisms (i.e. Trichoderma, Gliocladium, nitrifying bacteria) in the substrate (or substantially population) and may actually increase the incidence of soil-borne diseases. Unless field soil is added to a substrate, or there is some reason to believe the substrate may be contaminated with weed seed, nematodes or a high level of disease-causing organisms (i.e. with repeated monoculture in ground beds), substrates should not generally be pasteurized.
22.5.1 Methods of Substrate Pasteurization
22.5.1.1 Steam:
Steaming is the best methods for pasteurization of substrates. The basic objective is to apply the steam until the coldest area of the substrate reaches 160° F (71° C) for 30 minutes. Of course, other areas of the substrate will be at a higher temperature. The substrate should be moist and loose prior to steaming and a system that allows the steam to move into the substrate should be used.
Lime, superphosphate, inorganic fertilizers and microelements can be incorporated prior to steam pasteurization. However, slow-release fertilizers (unless otherwise indicated), manures and urea-based fertilizers should not be incorporated prior to pasteurization.
Several problems can occur as a result of steam pasteurization of substrates. Manganese toxicity can occur if a field soil containing large amounts of manganese is included in the substrate. Toxic levels of ammonium can occur when substrates with high volumes of organic matter, manures or ammoniacal nitrogen are pasteurized. This often occurs because the microorganisms involved in converting organic nitrogen to nitrate are killed by pasteurization. The ammonifying bacteria recover first followed 3- 6 weeks later by the nitrifying bacteria. During this time ammonium can build up to toxic levels. If slow release fertilizers are added prior to steam pasteurization, the coating may be damaged and fertilizer salts rapidly released. This can result in a high E.C. levels and subsequent damage to the plant root system.
22.5.1.2 Chemicals:
Methyl Bromide is scheduled to be removed from the market. It is highly toxic and is most often used for field situations. When being treated with methyl bromide, the soil should be loose and moist and must be covered tightly prior to the injection of the gas. Since methyl bromide is odourless, chloropicrin is usually added as a safety precaution. Methyl bromide effectively kills soil-borne fungal and bacterial pathogens, nematodes and weed seed.
Chloropicrin is also known as tear gas. It is used in a manner similar to methyl bromide.
Vapam is similar to chloropicrin. It is often used in the nursery industry to pasteurize soil beds. It is most effective against weed seed.
Growers should always exercise caution when using chemicals and follow the label for the specific chemical being used. After chemical sterilization, the medium should be allowed to sit for up to 14 days before planting to allow any phytotoxic chemical residue to dissipate.
22.5.2 Important facts of Root media Pasteurization
Greenhouse root media should be pasteurized at least once per year and more often as required to rid them to harmful disease organisms, nematodes, insects and weed seed.
Numerous microorganisms develop in root media which are not harmful. These can be beneficial by providing competition for harmful microorganisms, which might otherwise proliferate. For this reason root media are pasteurized and not sterilized i.e. only some organisms are killed.
Root medium may be pasteurized with steam by raising it to a temperature of 140° - 160°F for 30 minutes.
Volatile chemicals are also used for pasteurizing root media.
Both steam and chemical pasteurization require that the root medium be loose and of moisture content suitable for planting amendments such as peat moss, manure and bark should be incorporated prior to pasteurization to prevent introduction of diseases or pests.
Pasteurization can result in ammonium and manganese toxicities in certain situations. If the root medium contains organic matter rich in nitrogen, such as manure, steam and chemical pasteurization can result in an excessive release of ammonium, particularly in the period of two six weeks after pasteurization. Either these materials should be avoided or an adjustment should be made in the watering practice to ensure adequate leaching of ammonium. Many soils contain large levels of manganese, most of which is unavailable. Steam pasteurization causes conversion unavailable manganese to an available form. A toxic level is sometimes reached. This is another reason for pasteurizing root media at low temperature and for only the necessary length of time.
Pasteurization of root media is designed to eliminate harmful organisms. It does not protect against future infestations. Good sanitation practices must be employed to maintain clean conditions. Some considerations include disease free seeds and plants, sterilization of containers and tools, a pesticide program, foot baths, a clean working area, sanitation outside the greenhouse, and proper control of temperature and humidity.
REFERENCES
1. Thomas M. Blessington et.al. “Methods of watering Greenhouse crops.” Fact sheet 867, Maryland Cooperative Extension, University of Maryland
2. Nicolas Castilla, 2013. “Greenhouse Technology and Management.” Ediciones Mundi-Prensa, Madrid (Spain) and Mexico.PP.179-201