Site pages
Current course
Participants
General
Module 1. History and types of greenhouse
Module 2.Function and features of greenhouse
Module 3.Scope and development of greenhouse techn...
Module 4.Location, planning and various components...
Module 5.Design criteria and calculations
Module 6. Construction materials and methods of co...
Module 7. Covering material and characteristics
Module 8. Solar heat transfer
Module 9. Solar fraction for greenhouse
Module 10. Steady state analysis of greenhouse
Module No. 11 Greenhouse Heating, Cooling, Sheddin...
Module 12. Carbon dioxide generation and monitorin...
Module 13. Instrumentation and & computerized ...
Module 14. Watering, fertilization, root substrate...
Module 15. Containers and benches
Module 16. Plant nutrition, Alternative cropping s...
Module 17. Plant tissue culture
Module 18. Chemical growth regulation
Module 19. Disease control, integrated pest manage...
Module 20: Post Production Quality and Handling
Module 21: Cost analysis of greenhouse Production
Module 22. Application of greenhouse & its rep...
Lesson 24. Plant Nutrition and Alternative Cropping System
24.1 INTRODUCTION
This chapter deals with the basics of developing a nutritional program for producing container-grown plants in greenhouses. A complete nutrition program encompasses the fertilizers, media and water used. The information about the plant nutrition and range of fertilizers available that growers need to understand will be discussed. Also alternative cropping system other than traditional systems such as hydroponic cropping technology will be discussed.
24.2 PLANT NUTRITION: THE BASICS
24.2.1 Fertilizer Salts
Fertilizers are salts. Salts are chemical compounds that contain one positively charged ion (cation) bonded to one negatively charged ion (anion). When a salt is placed into water, the two ions separate and dissolve. An example of a fertilizer salt is calcium nitrate, which contains one calcium cation and a nitrate anion. Other examples include: ammonium phosphate, magnesium sulphate, potassium nitrate and ammonium nitrate.
Fertilizer concentration (or saltiness) of a solution can be determined by measuring the ability of a solution to conduct an electrical signal (electrical conductivity). Electrical conductivity meters, often called soluble salts meters, measure the concentration of salts/ions in solution; therefore, a grower can always measure the amount of fertilizer being applied to a crop. However, electrical conductivity meters do not specifically measure which specific salts are in solution. For example, an electrical conductivity meter cannot tell the difference between table salt (sodium chloride), which is dangerous to plants, and potassium nitrate, which is useful for plants. Ions dissolved in water are taken up through the roots and distributed within the plant. Plants actually expend energy to take up most ions, however, calcium is thought to only come along for the ride, i.e., plants don’t actively take up calcium, it just comes into the root with the water.
Once inside the plant, ions are recombined into compounds useful for plant growth. The most common example of plant metabolism involves photosynthesis, during which water (hydrogen and oxygen) is combined with carbon dioxide (carbon and oxygen) to form starch or sugars (carbon, hydrogen and oxygen). Another example is the chlorophyll molecule shown below that contains:
55 carbon atoms
60 hydrogen atoms
5 oxygen atoms
4 nitrogen atoms
1 magnesium atom
Therefore, for the plant to build one chlorophyll molecule, the leaves must take in carbon dioxide, for the carbon and oxygen; the roots must take in water, for the hydrogen and oxygen; and the roots must take nitrogen and magnesium provided from the fertilizer applied.
Once inside the plant, some nutrients can be mobilized to support new growing tissues, while other nutrients are fixed in older plant tissues. This fact helps us to diagnose some plant nutrient deficiencies. For example, if a plant is deficient in an immobile nutrient, then deficiency symptoms (yellowing/chlorosis) occur in the new growth, since the older tissues “hold” on to the immobile nutrients. In contrast, deficiencies of mobile nutrients typically occur in the older leaves, since the mobile nutrients move from the old leaves to the new leaves.
Plants require different amounts of each nutrient. Carbon, hydrogen and oxygen are required in the greatest amounts; however, these are taken up by the plant in the form of water and carbon dioxide. Nitrogen, phosphorus, potassium, calcium, magnesium and sulphur are required in large amounts, thus are called macronutrients. Iron, manganese, copper, zinc, boron, chloride, molybdenum are required in relatively small amounts, thus are called micronutrients, or minors.
24.2.2 pH
pH is a measure of the concentration of hydrogen (H+) ions, also called protons. The greater the H+ ion concentration, more acidic the solution, hence a lower pH. pH controls the uptake of nutrients. If the pH is not in the desired range, individual nutrients cannot be taken up, creating a nutrient deficiency, or the nutrient can be taken up too readily, resulting in a nutrient toxicity. These nutrient imbalances will occur even when proper amounts of nutrients are applied to the media, if the pH is too high or too low. Nitrogen and potassium are readily available at a wide pH range. Although phosphorus is more readily available at a low pH, phosphorus problems are not commonly observed in greenhouse crops. Calcium and magnesium are more readily available at a higher pH. At a low pH, the minor nutrients (iron, manganese, boron, zinc and copper) are readily available. Minor nutrient toxicities are relatively common at a low pH (< 5.8), while deficiencies frequently occur at a high pH (> 6.5).
24.2.3 Factors Affecting Media Solution pH:
1. Water Quality/Alkalinity: Alkalinity is one measure of the quality of water used for irrigation. Alkalinity is the measure of the concentration of bicarbonates and carbonates in water which determine the water’s capacity to neutralize acids. In other words, irrigating with bicarbonates in water is equivalent to applying lime every time through irrigation. The bicarbonates react with hydrogen ions and remove them from solution. This process effectively decreases the H+ ion concentration in the media and thus increases the media solution pH. The reverse situation can also occur. Very pure water (low bicarbonates) can cause media solution pH to decrease over time. The pH drops, because there may not be enough bicarbonates to absorb excess hydrogen ions. Thus, the H+ ion concentration in the media increases. The most common solution for pure water sources is to increase the amount of pulverized dolomitic limestone incorporated into the media prior to transplanting plants into the media. Another solution is to top-dress containers with the limestone. Finally, bicarbonate can be added to irrigation water in the form of potassium bicarbonate to improve the buffering capacity of the media solution (i.e., reduce pH fluctuation).
2. Media Component: Peat tends to be acidic. Pulverized dolomitic limestone (CaMg (CO3)2) is incorporated into most amended media to adjust the starting pH to ~6.0. Coarser grades of dolomitic limestone change the media pH more slowly, and thus are not often used in peat-based media. A relatively new, but popular media component, coconut coir, is less acidic than peat, so less limestone needs to be used.
3. Fertilizers Applied: Fertilizers are categorized into one of two groups: acid-residue or alkaline-residue. The fertilizers themselves are not acidic or alkaline, but they react with microorganisms in the media and plant roots to affect media solution pH. Fertilizers with ample ammonium or urea tend to acidify the media, i.e., lower the pH. Fertilizers with ample nitrates tend to raise the pH of the media solution slowly over time.
24.3 Fertilizers and Fertilization
24.3.1 Water Soluble Fertilizers
Most greenhouse fertilization programs rely on water-soluble fertilizers to provide most of the nutrients required for plant growth. Water-soluble fertilizers are often applied at the each irrigation. This is referred as a specific fertilizer program must be developed around the irrigation water, media and crops grown.
The 20-10-20 Peat-Lite Special supplies nitrogen, phosphorus, potassium and minor nutrients. The 15-0-15 fertilizer supplies nitrogen, potassium, calcium and minor nutrients. The epsom salts supply magnesium and sulphur. Rotating these three products provides all essential nutrients required for plant growth. Recently available are water-soluble fertilizers that supply all essential nutrients in one fertilizer. Examples include 15-5-15 Cal-Mag Special and 13-2-13 Plug Special.
24.3.2 Slow-Release Fertilizers
Slow-release, or controlled-release, fertilizers are usually used when crops are grown outdoors. Slow-release fertilizers are beneficial because they create less environmental pollution, e.g., fertilizer run-off, when sprinkler irrigation is used, and they continue to supply nutrients during rainy weather. Slow-release fertilizers are marketed based on the time of release, for example, 3 to 4 month longevity. The actual fertilizer release rate is determined by the temperature and water content of the media. Therefore, the actual effective release time of the fertilizer may vary from the labelled time. Slow release fertilizers can be incorporated into the media prior to filling the containers or top dressed after planting.
24.3.3 Fertilizer Labels
24.3.3.1 Nutrient analysis
The fertilizer analysis indicates the percentage of a particular nutrient contained within the fertilizer (on a percent weight basis). The fertilizer analysis typically refers to the percentage of nitrogen (N), phosphate (P2O5) and potash (K2O) contained in a given fertilizer. A balanced fertilizer should provide nutrients in amounts relative to plant requirements. Since nitrogen and potassium are used in relatively similar amounts (on a weight basis), a fertilizer should have a nitrogen to potassium ratio of approximately 1:1. Phosphorus is required to a lesser degree, so the nitrogen-to-phosphorus ratio should be approximately 2:1 to 4:1. Therefore, a 2:1:2 (N-P2O5-K2O) is suitable for most greenhouse crops. An example of this type of fertilizer is 20-10-20. While 20-20-20 is still commonly used, 20-10-20 is preferred, since the N-P2O5-K2O ratio is closer to that required by plants. The extra phosphorus provided by 20-20-20 is usually wasted, thus creating potential environmental concerns.
24.3.3.2 Nitrogen Form.
Nitrogen is provided in three different forms: nitrate-nitrogen (NO3), ammoniacal-nitrogen (NH4) and urea-nitrogen. The nitrogen form affects plant growth and media solution pH. Ammoniacal nitrogen, sometimes called ammonium, tends to contribute to “lush” plant growth, for example, greater leaf expansion and stem elongation, whereas nitrate nitrogen produces a “hard” or well-toned and compact plant. High ammonium can be toxic to plants during cold, cloudy growing conditions. Therefore, ammonium and urea should make up less than 40 percent of the nitrogen during winter months. “Dark-Weather” or “Finisher” fertilizers tend to have high nitrate and low ammonium nitrogen.
The following equation demonstrates how to calculate the percentage of the total nitrogen that is in the ammoniacal form.
% N in ammonium form = (% Ammonium + % Urea) ÷ % Total N ×100
For example, a 15-5-15 label indicates the following nitrogen breakdown:
Total Nitrogen (N) = 15%,
Ammoniacal Nitrogen = 1.2 %,
Nitrate Nitrogen =11.75 %,
Urea Nitrogen = 2.05 %
(2.05% Urea + 1.20% Ammonium)÷ 15% Total N × 100 = 21.7% N in ammonium form
24.3.3.3 Potential acidity or basicity
The potential acidity or basicity indicates how the fertilizer will affect media solution pH. A fertilizer label will indicate that the fertilizer has either a potential acidity or a potential basicity. The potential acidity refers to the fertilizer’s tendency to cause the media pH to decrease, while the potential basicity refers to the fertilizer’s tendency to cause a media pH increase. Fertilizers high in ammonium cause the pH to decrease (become more acidic), while fertilizers high in nitrate cause the pH to increase (become more basic or alkaline). Fertilizers with a considerable percentage of the nitrogen in the ammonium form tend to leave an acid residue in the media, indicated by the potential acidity. Fertilizers that have low ammonium, and thus high nitrate form of nitrogen, tend to leave an alkaline, residue indicated by the potential basicity. The high-ammonium fertilizers tend to have very little or no calcium or magnesium, while the low-ammonium, alkaline-residue fertilizers contain higher levels of calcium or magnesium.
24.3.3.4 Proper Dilution Rate: The proper dilution rate is indicated on the fertilizer label and can be tested with a soluble salts meter. The soluble salts concentration of the fertilizer solution increases as the amount of fertilizer increases. For example, 20-10-20 Peat-Lite Special will have an electrical conductivity (EC) of 0.33 mmhos/cm for every 50 ppm of nitrogen. Therefore, a fertilizer mixed to provide 250 ppm nitrogen will have an EC of 1.65 mmhos/cm [(250÷ 50) × 0.33=1.65].
24.3.4 Fertilizer injectors
Injectors mix precise volumes of concentrated fertilizer solution and water together. Injectors are commonly available in a mixing range of 1:16 to 1:200. For example, 1:100 injection ratio indicates that one gallon of concentrated fertilizer will produce 100 gallons of final fertilizer solution. Injectors allow growers to have a smaller stock tank and mix their fertilizer stock solutions less frequently. However, not all fertilizers can be mixed together. Calcium and magnesium fertilizers typically cannot be mixed with phosphate and sulphate fertilizers while concentrated. A solid precipitate will form in the bottom of the stock tank if the fertilizers are not compatible. Once the individual fertilizers are diluted to their final concentration, then all fertilizers are compatible and thus can be mixed together.
24.3.5 Multiple Injectors.
Multiple injectors or multiple-headed injectors can be used to inject incompatible stock solutions. If separate injectors are plumbed serially, i.e., one after the other, then fertilizer stock solutions can be mixed at the same concentration as if one injector is being used. For example, one head can inject calcium nitrate, while the other head injects magnesium sulphate. However, if two injector heads are placed into one stock solution, then the final con- centration delivered to the plants will be twice the desired concentration, unless proper dilution occurs, e.g., mix the stock solution for 100 ppm N if 200 ppm is desired.
24.4 STARTING A FERTILIZATION PROGRAM
Nutrients can be placed into the media prior to planting, i.e., a pre-plant nutrition program, and during plant growth, i.e., a post-plant nutrition program. Do not forget that irrigation water can also be a significant source of plant nutrients, especially calcium and magnesium.
Despite considerable gardening advice to the contrary, specific nutrients do not promote rooting or flowering! Specifically, phosphorus does not promote rooting and potassium does not promote flowering. Excess nitrogen can potentially reduce flowering and produce excessive vegetative growth.
24.4.1 Pre-Plant Nutrition Programs
Nutrients can be supplied in limited quantities, while the media components are being mixed. Calcium and magnesium are provided when dolomitic limestone is used to adjust the starting pH. Phosphorus and sulphur are provided with superphosphate plus gypsum (calcium and sulphur). (Single
phosphate is 50 percent gypsum by weight). Iron, manganese, zinc, copper, boron and molybdenum are provided with micronutrient formulations. Nitrogen and potassium are provided with potassium nitrate. Typical pre-plant recipe for 1 cubic yard of soilless media:
|
Dolomitic limestone |
10 lbs. |
|
Treble Superphosphate |
2.25 lbs |
|
Gypsum |
1.5 lbs. |
|
Micromax |
1.25 lbs. |
|
Potassium Nitrate |
1 lb. |
24.4.2 Post Plant Nutrition Programs
Most small to medium sized commercial greenhouses use commercially blended fertilizers for convenience and dependability; however, for some growers it is economical to buy individual fertilizers and mix them together. Following are some important notes about each of the essential plant nutrients:
24.4.2.1 Nitrogen (N)
Sources: ammonium nitrate, urea, calcium nitrate, potassium nitrate, magne- sium nitrate.
The concentration applied is determined by the amount of leaching. For example, in a constant liquid feed program using a sub-irrigation system (0 % leaching) 100 ppm N may produce adequate growth, while 300 ppm N may be needed if overhead irrigation results in 25 percent leaching.
24.4.2.2 Phosphorus (P)
Sources: Ammonium phosphate, urea phosphate.
A nitrogen-to-phosphate (P2O5) ratio of 2:1 is acceptable for most crops. Fertilizers with high concentrations of ammonium phosphate, such as 9-45-15, appear to promote stem stretching.
24.4.2.3 Potassium (K)
Sources: Potassium nitrate, potassium sulphate
A nitrogen-to-potash (K2O) ratio of 1:1 is acceptable for most crops.
24.4.2.4 Calcium (Ca) and Magnesium (Mg)
Sources: Dolomitic limestone, irrigation water, calcium nitrate, magnesium sulphate (Epsom salts), magnesium nitrate.
Calcium and magnesium provided by dolomitic limestone are released slowly over several months. These two nutrients can have an antagonistic relationship (i.e., they compete within the plant), thus a Ca: Mg ratio of 3:1 to 5:1 is desirable. Calcium and magnesium are commonly found in irrigation water, especially high alkalinity water. Calcium and magnesium deficiencies are most common when the pH is low (less than 5.8). Calcium and magnesium fertilizers cannot be mixed in the concentrated form with phosphate or sulphate fertilizers, thus calcium and magnesium are frequently omitted from commercial fertilizers. A few relatively new fertilizers contain calcium and magnesium along with the nitrogen, phosphorus and potassium. These fertilizers often list five numbers in the analysis. These numbers represent N, P2O5, K2O, Ca and Mg, respectively.
24.4.2.5 Micronutrients
Micronutrients are sold in different formulations; for example, Micromax, Esmigran and Soluble Trace Element Mix contain only inorganic sources, while Compound 111 contains chelated sources. Chelated forms are superior in that the micronutrients are more soluble, therefore more readily available to the plant. Consequently, chelated micronutrients are applied at lower rates. Compound 111 and STEM are labelled for use in constant liquid feed programs.
The rates are based on adding a certain amount of micronutrient mix per 100 ppm of N used in the fertilization program.
Micronutrient deficiencies are closely related to media pH. High pH (greater than 6.5) can produce deficiencies, while low pH (less than 5.8) can cause toxicities. Adjusting the media pH is the best solution to avoid micronutrient toxicities or deficiencies.
24.5 FERTILIZER CALCULATION
Calculate the parts per million for a fertilizer application
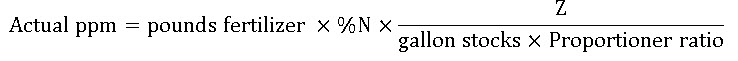
For N, Ca, Mg, Fe, Z=1200
For Phosphorus (P), Z= 528
For Potassium (K), Z= 996
- Calculate the concentration (ppm) of nitrogen applied when 1 pound of 15-0-15 is mixed into a 5 gallon stock tank and a 1:16 ratio is used.
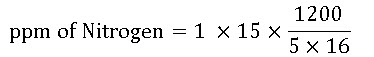
= 225 ppm
2. Calculate the concentration (ppm) of potassium applied when 1 pound of 15-0-15 is mixed into a 5 gallon stock tank and a 1:16 ratio is used.
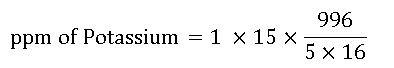
= 186.75 @ 187 ppm
3. Calculate the concentration (ppm) of calcium applied when 1 pound of 15-0-15 is mixed into a 5 gallon stock tank and a 1:16 ratio is used. (Note: 15-0-15 contains 11% calcium). Also note that the irrigation water has 15 ppm calcium.
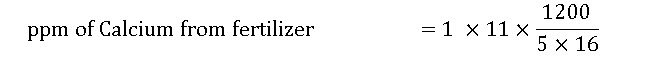
= 165 ppm
Additional ppm of calcium from water = 15 ppm
Hence, Total calcium applied = 165 + 15=180 ppm
24.6 ALTERNATIVE CROPPING SYSTEM
24.6.1 Hydroponic Technology
Soilless (Hydroponic) culture which was initially developed for studying plant mineral nutrition is thought to be one of the main elements of sustainable cropping systems under greenhouse conditions. In fact implementation of closed hydroponics may reduce drastically the use of water and fertilizers and the environmental pollution associated to over irrigation which is quiet common in protected horticulture. However, the application of closed loop hydroponic technology is scarce on commercial scale. The main technical features of the hydroponic technology are illustrated below.
24.6.1.1 Technology
Hydroponic is broad term that includes all techniques for growing plants in media other than soil or in aerated nutrient solution. The classification of soilless culture considers the type of substrate and container, how the nutrient solution is delivered to the plant (drip irrigation, subirrigation, flowing, stagnant or mist nutrient solution culture) and the fate of the drainage nutrient solution: open (free drain) or closed (recirculating water) systems. The most widely used soilless technics are drain to waste substrate cultivation, while water cultivation systems such as nutrient film technique (NFT), floating culture and aeroponics are widely used for research work but much less on commercial scale.
Table 24.6.1 Characteristics of various hydroponic techniques
|
|
Substrate and drip irrigation |
Substrate and subirrigation |
NFT |
Floating system |
Aeroponics |
|
Application for commercial production |
Large |
Large |
Scarce |
Increasing |
Rare |
|
Type of crops |
Fruit vegetables Strawberry Cut flowers |
Pot plants |
Leafy vegetables |
Leafy vegetables Bulb flowers |
Vegetables |
|
Growing media |
Yes (Organic/inert) |
Yes (organic) |
No |
No |
No |
|
Recirculating solution |
Yes/no |
Yes |
Yes |
Stagnant or fairly static |
Yes |
|
Investment costs |
Moderate/high |
High |
High |
Low |
Very high |
|
Running costs |
Moderate/high |
Moderate/ high |
Moderate |
Low |
Fair/high |
|
System’s buffer |
High |
High |
Low |
High |
Very low |
|
Growing risks |
Moderate |
Moderate |
High |
Moderate |
Very high |
24.6.1.2 Substrate Culture
Substrate culture is generally used for row crops such as fruit vegetables, strawberry and cut flower. Different containers (Figure 24.6.1) such as banquette, pots, bags, slabs are used filled with inorganic or organic substrate or a combination of two or three different materials such as peat perlite or peat pumice mixture. An excess of nutrient solution is typically supplied to the crop by drip irrigation. In the cultivation of pot ornamentals, sub irrigation is increasingly adopted; the posts are cultivated in gullies with an intermittent flow of nutrient solution or in ebb and flow benches. Both open and closed system may be set up for drip irrigated substrate culture. In closed system the drainage water is captured and reused following the adjustment of pH and nutrient concentration and eventually disinfection to minimize the risk of root- borne diseases (Fig 24.6.2).

Fig 24.6.1 Long cycle tomato culture in pertile bags in a greenhouse
(Source: Giuliano Vox et al.2010)
Peat is largely used for pot ornamentals and propagation materials (seedlings, cuttings and micro propagated plantlets). In mixture with perlite it is largely used for bag culture of strawberry (fig 24.6.3). The most popular growing media for growing row crops are perlite and rock-wool, which are easy to handle, sterilise and re-use for few years.

Fig 24.6.3 Suspended bag culture of strawberry
(Source: Giuliano Vox et al.2010)
24.6.1.3 Water Culture
The most used water culture methods are floating raft systems, NFT, aeroponics. These are closed systems.
24.6.1.3.1 Floating rafts growing technology (Fig. 24.6.4) is certainly the most water conscious system among existing hydroponic growing systems. One of its key feature is the use of a large volume of water allowing enormous buffer for fertilization and oxygen control and economic plants' transportation by flotation. This large buffer brings a level of security and easiness that no other growing system can match. This system is mostly used for leafy vegetables, herbs, bulb flowers.

Fig 24.6.4 Floating raft system
(Source: www.randyshydroponics.com)
24.6.1.3.2 Nutrient film technique (NFT)(Fig. 24.6.5) is a hydroponic technique wherein a very shallow stream of water containing all the dissolved nutrients required for plant growth is re-circulated past the bare roots of plants in a watertight gully, also known as channels. In an ideal system, the depth of the recirculating stream should be very shallow, little more than a film of water, hence the name 'nutrient film'. This ensures that the thick root mat, which develops in the bottom of the channel, has an upper surface, which, although moist, is in the air. Subsequent to this, an abundant supply of oxygen is provided to the roots of the plants. A properly designed NFT system is based on using the right channel slope, the right flow rate, and the right channel length. The main advantage of the NFT system over other forms of hydroponics is that the plant roots are exposed to adequate supplies of water, oxygen and nutrients. NFT, because of its design, provides a system wherein all three requirements for healthy plant growth can be met at the same time, provided that the simple concept of NFT is always remembered and practiced. The result of these advantages is that higher yields of high-quality produce are obtained over an extended period of cropping. A downside of NFT is that it has very little buffering against interruptions in the flow, e.g., power outages, but, overall, it is one of the more productive techniques.
The same design characteristics apply to all conventional NFT systems. While slopes along channels of 1:100 have been recommended, in practice it is difficult to build a base for channels that is sufficiently true to enable nutrient films to flow without ponding in locally depressed areas. As a consequence, it is recommended that slopes of 1:30 to 1:40 be used. This allows for minor irregularities in the surface, but, even with these slopes, ponding and water logging may occur. The slope may be provided by the floor, or benches or racks may hold the channels and provide the required slope. Both methods are used and depend on local requirements, often determined by the site and crop requirements.
The high installation costs, the small buffering capacity and some still unresolved problems, like those related to both non parasitic and parasitic diseases of root system, have hampered the commercial application of NFT, which are generally used for short season crops.
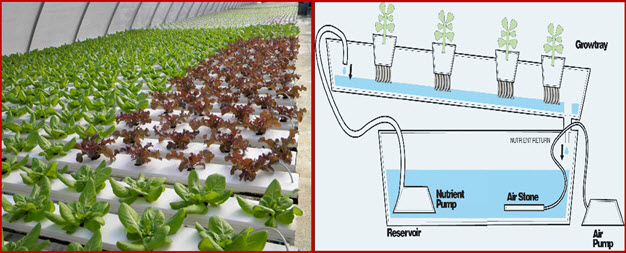
Fig 24.6.5 NFT system
(Source: www.hydroponicphd.wordpress.com , www.cropking.com )
24.6.1.3.3 Aeroponics (Fig.24.6.6) is the process of growing plants in an air or mist environment without the use of soil or an aggregate medium (known as geoponics). The word "aeroponic" is derived from the Greek meanings of aero- (air) and ponos (labour). Aeroponic culture differs from conventional hydroponics, aquaponics, and in-vitro (plant tissue culture) growing. Unlike hydroponics, which uses a liquid nutrient solution as growing medium and essential minerals to sustain plant growth; or aquaponics which uses water and fish waste, aeroponics is conducted without a growing medium. Because water is used in aeroponics to transmit nutrients, it is sometimes considered a type of hydroponics.
The basic principle of aeroponic growing is to grow plants suspended in a closed or semi-closed environment by spraying the plant's dangling roots and lower stem with an atomized or sprayed, nutrient-rich water solution. The roots of the plant are separated by the plant support structure. Many times closed cell foam is compressed around the lower stem and inserted into an opening in the aeroponic chamber, which decreases labour and expense; for larger plants, trellising is used to suspend the weight of vegetation and fruit.

Fig 24.6.6 Aeroponics system
(Source: www.farmxchange.org)
24.6.1.3.3.1 Types of aeroponics
A. Low-pressure units
In most low-pressure aeroponic gardens, the plant roots are suspended above a reservoir of nutrient solution or inside a channel connected to a reservoir. A low-pressure pump delivers nutrient solution via jets or by ultrasonic transducers, which then drips or drains back into the reservoir. As plants grow to maturity in these units they tend to suffer from dry sections of the root systems, which prevent adequate nutrient uptake. These units, because of cost, lack features to purify the nutrient solution, and adequately remove continuities, debris, and unwanted pathogens. Such units are usually suitable for bench top growing and demonstrating the principles of aeroponics.
B. High-pressure devices
High-pressure aeroponic techniques, where the mist is generated by high-pressure pump(s), are typically used in the cultivation of high value crops and plant specimens that can offset the high setup costs associated with this method of horticulture. High-pressure aeroponics systems include technologies for air and water purification, nutrient sterilization, low-mass polymers and pressurized nutrient delivery systems.
C. Commercial systems
Commercial aeroponic systems comprise high-pressure device hardware and biological systems. The biological systems matrix includes enhancements for extended plant life and crop maturation.
Biological subsystems and hardware components include effluent controls systems, disease prevention, pathogen resistance features, precision timing and nutrient solution pressurization, heating and cooling sensors, thermal control of solutions, efficient photon-flux light arrays, spectrum filtration spanning, fail-safe sensors and protection, reduced maintenance & labour saving features, and ergonomics and long-term reliability features.
Commercial aeroponic systems, like the high-pressure devices, are used for the cultivation of high value crops where multiple crop rotations are achieved on an ongoing commercial basis.
Advanced commercial systems include data gathering, monitoring, analytical feedback and internet connections to various subsystems.
REFERENCES
1. James U. Fast and Elizabeth Will, “Plant Nutrition and Fertilizers for Greenhouse Productions” PB1616, Agricultural Extension Service, University of Tennessee.
2. Giuliano Vox et.al., (2010). “Sustainable Greenhouse Systems.” Sustainable Agriculture, pp-1-79.