Site pages
Current course
Participants
General
Module 1. History and types of greenhouse
Module 2.Function and features of greenhouse
Module 3.Scope and development of greenhouse techn...
Module 4.Location, planning and various components...
Module 5.Design criteria and calculations
Module 6. Construction materials and methods of co...
Module 7. Covering material and characteristics
Module 8. Solar heat transfer
Module 9. Solar fraction for greenhouse
Module 10. Steady state analysis of greenhouse
Module No. 11 Greenhouse Heating, Cooling, Sheddin...
Module 12. Carbon dioxide generation and monitorin...
Module 13. Instrumentation and & computerized ...
Module 14. Watering, fertilization, root substrate...
Module 15. Containers and benches
Module 16. Plant nutrition, Alternative cropping s...
Module 17. Plant tissue culture
Module 18. Chemical growth regulation
Module 19. Disease control, integrated pest manage...
Module 20: Post Production Quality and Handling
Module 21: Cost analysis of greenhouse Production
Module 22. Application of greenhouse & its rep...
Lesson 26 Plant Tissue Culture: Basic Process and Techniques Used
26.1 INTRODUCTION
Plant tissue culture may be defined as in vitro (in glass vessels) culture of an explant (any plant part used to initiate in vitro culture; e.g. shoot tip, leaf, petiole etc.),under aseptic conditions (sterile; free from microorganisms), under controlled environment (uniform temperature, humidity, light duration etc.), on a specific medium (which provides nutrient for plant growth and usually contains one or more plant growth regulators), for a specific purpose (e.g. for mass multiplication, genetic transformation, production of disease free plants, etc.).
Totipotency forms the basis of successful plant tissue culture. The theory of Totipotency states that each cell has the ability to regenerate into a complete plant. Each somatic cell has the same genetic constitution (DNA sequence) as that of a zygote, and hence, also has the potential of expressing all the properties of an organism. Since, handling a single cell is practically difficult, therefore, usually a tissue or an organ form the plant is used to initiate the tissue culture work and hence Plant Tissue Culture is often also called as Plant Cell, Tissue and Organ Culture.
26.2 THE BASIC STEPS INVOLVED IN PLANT TISSUE CULTURE
A flow chart of the basic steps involved in plant tissue culture is given in Fig. 26.1.
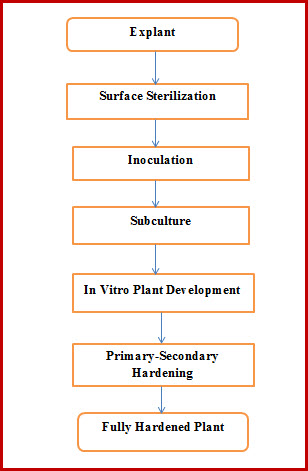
Fig 26.1 The Basic Steps Involved in Plant Tissue Culture
The following is a brief description of the tissue culture process and some terminologies:
26.2.1 Explant:
Any plant part used to initiate the tissue culture is known as an Explant. Theoretically any plant part may be used as an explant, however, the selection of an explant largely depends on the purpose of doing tissue culture, suitability of an explant for initiating cultures and availability of an explant.
26.2.2 Sterilization:
Sterilization is the process of making any thing free from microorganisms (i.e. aseptic). Since the process of tissue culture involves culturing plants on a nutrient rich medium, proper sterilization is a pre-requisite. The sterilization processes vary with the material to be sterilized.
Explant à Surface sterilization à Chemical Treatment (Ref. Table 1)
Media à Autoclaving (steam sterilization, at 121°C and 15 psi)
Heat labile PGRs / Hormones à Filter sterilization (0.22 μm filter)
Air à HEPA filters / Lamina air flow units
Forceps / blade handles & other tools à Flame sterilization
Rooms à Fumigation & UV radiation
Laminar Airflow hood à UV radiation
Table 1: Commonly used disinfectants for surface sterilization of the explant in plant tissue culture:
|
Sr. No. |
Disinfectant |
Concentration (%) |
Exposure (min) |
|
Calcium hypochlorite |
9-10 |
5-30 |
|
|
Sodium hypochlorite* |
0.5-5 |
5-30 |
|
|
Hydrogen peroxide |
3-12 |
5-15 |
|
|
Ethyl alcohol |
70-95 |
0.1-5.0 |
|
|
Silver nitrate |
1 |
5-30 |
|
|
Mercuric chloride |
0.1-1.0 |
2-10 |
|
|
Benzalkonium chloride |
0.01-0.1 |
5-20 |
*Commercial bleach contains about 5% sodium hypochlorite, and thus may be used at a concentration of 10-20%, which is equivalent to 0.5-1.0% sodium hypochlorite.
26.2.3 Inoculation:
Placement of an explant (after surface sterilization) on a sterilized establishment medium under aseptic conditions.
26.2.4 Subculture:
Transfer of the growing/ multiplying explant/(s) from one medium to another under aseptic conditions. It is usually done at an interval of 21 days, as the media usually gets used up for plant growth within this duration or to change the course of plant growth.
26.2.5 Media:
The MS medium of Murashige and Skoog (1962) salt composition is very widely used in different culture systems. It was demonstrated that not only the presence of necessary nutrients but also the actual and relative concentrations of various inorganic nutrients are of crucial significance. Any success with a medium is due to the fact that the ratios as well as concentrations most nearly match the optimum requirements for the cells or tissues for growth and/or differentiation.
The nutritional medium generally consists of inorganic nutrients, carbon and energy sources, vitamins, phytohormones (growth regulators), and organic supplements which include organic nitrogen, acids and complex substances.
26.2.6 Inorganic nutrients
Mineral elements are very important in the life of a plant. For example, calcium is a component of the cell wall, nitrogen is an important part of amino acids, proteins, nucleic acids, and vitamins, and magnesium is a part of chlorophyll molecules. Similarly iron, zinc and molybdenum are parts of certain enzymes. Besides, C, H, N, and 0, 12 other elements are known to be essential for plant growth. If the elements required by plants in concentration greater than 0.5 mmol/l are referred to as macro-elements and those required in concentration less than that are microelements. The example of macro are N, K, P, Ca, S, and Mg and the microelements are iron (Fe), manganese (Mn), boron (B), copper (Cu), zinc (Zn), iodine (I), molybdenum (Mo) and cobalt (Co). For most purposes a nutrient medium should contain from 25 to 60 mM inorganic nitrogen. Nitrate is used in the range of 25-40 mM and ammonium in the range of 2-20 mM.
26.2.7 Carbon and energy source
Without exception, the standard carbon source is sucrose or glucose. Fructose can also be used but is less efficient. The sucrose in the medium is rapidly converted into glucose and fructose. Glucose is then utilized first, followed by fructose. Sucrose is generally used at a concentration of 2 -5%. Other carbohydrates which have been tested include lactose, maltose, galactose and starch, but these compounds are generally much inferior to sucrose or glucose. Most media contain myo-inositol at a concentration of ca. 100 mg/l, which improves cell growth.
26.2.8 Vitamins
Normal plants synthesize the vitamins required for growth and development. But plant cells in culture have a requirement for vitamins. There is an absolute requirement for vitamin B1 (thiamine). Growth is also improved by the addition of nicotinic acid (B4)and vitamin B6 (pyridoxine). Some media contain pantothenic acid, biotin, folic acid, p-amino benzoic acid, choline chloride, riboflavin ( B2) and ascorbic acid ( Vit-C).
26.2.9 Growth regulators
Hormones are organic compounds naturally synthesized in higher plants, which influence growth and development. They are usually active at a site different from where they are produced and are only present and active in very small quantities. Apart from natural compounds, synthetic compounds have been developed which correspond to the natural ones. These are collectively called growth regulators. There are two main classes of growth regulators that are of special importance in plant tissue culture. These are the auxins and cytokinins, while the others viz. gibberellins, abscisic acid (ABA), ethylene, etc. are of. minor importance. Some of the naturally occurring hormones are the auxin - indole acetic acid (IAA) and the cytokinin - zeatin, while the others are synthetic growth regulators.
26.2.9.1 Auxins
A common feature of auxins is their property to induce cell division and formation of callus. Auxin causes cell division, cell elongation and swelling of tissues, and the formation of adventitious roots. It often inhibits adventitious and axillary shoot formation. At low auxin concentrations, adventitious root formation predominates, whereas at high auxin concentrations, root formation fails to occur and callus formation takes place. The compound most frequently used and highly effective is 2,4-dichlorophenoxy acetic acid (2,4-D). Other auxin in use include naphthalene acetic acid (NAA), Indole acetic acid (IAA), indole butyric acid (IBA), 2,4,5- trichlorophenoxy acetic acid (2,4,5 - T), p-chlorophenoxy acetic acid (pCPA) and picloram (4-amino-3,5,6-trichloropicolinic acid).
26.2.9.2 Cytokinins
Cytokinins are derivatives of adenine and have an important role in shoot induction. The compounds that are most frequently used are kinetin, benzyl adenine (BA) or 6-benzyl amino purine (BAP), zeatin, and isopentenyl adenine (2iP). These are often used to stimulate growth and development. They usually promote cell division, if added together with an auxin. At higher concentrations (1 to 10 mg / l), adventitious shoot formation is induced but root formation is generally inhibited. They promote axillary shoot formation by decreasing apical dominance. Stock solutions of IAA and kinetin are stored in amber bottles or bottles covered with a black paper and kept in dark since they are unstable in light.
26.2.9.3 Other hormones
Gibberellins are normally used in plant regeneration. GA3 is essential for meristem culture. Gibberellins induce elongation of internodes and the growth of meristems or buds in vitro. Gibberellins usually inhibit adventitious root as well as shoot formation.
Abscisic acid is an important growth regulator for induction of embryogenesis. Ethylene is I produced by cultured cells, but its role in cell and organ culture is not known.
26.2.10 Organic supplements
26.2.10.1 Organic nitrogen
Cultured cells are normally capable of synthesizing all the required amino acids, but it is often beneficial to include organic nitrogen in the form of amino acids such as glutamine and aspargine and nucleotides such as adenine. For cell culture, it is good to add 0.2 to 1.0 g/l of casein hydrolysate or vitamin-free casamino acids. The amino acids when added should be used with caution, since they can be inhibitory. The amino acids included in the media and amount in mg / I are: glycine (2), aspargine (100), tyrosine (100), arginine (10) and cysteine (10). Sometimes adenine sulphate (2-120 mg/l) is added to the agar for morphogenesis.
26.2.10.2 Organic acids
Plant cells are not able to utilize organic acids as a sole carbon source. Addition of TCA cycle acids such as citrate, malate, succinate or fumarate permits growth of plant cells on ammonium as the sole nitrogen source. The cells can tolerate a concentration of upto 10 mM of the acid.
26.2.11 Complex substances
A variety of extracts viz. protein hydrolysate, yeast extract, malt extract, coconut milk, orange and tomato juice have been tested. With the exception of protein hydrolysate and coconut milk, most others are used as the last resort. Coconut milk is commonly used at 2 -15% (v/v). The present trend is, however, towards fully defined media and the use of complex mixtures is losing favour.
Activated charcoal at concentrations of 0.2 to 3.0% (w/v) is used where phenol-like compounds are a problem for growth of cultures. It can adsorb toxic brown/black pigments and stabilizes pH. Besides activated charcoal, polyvinylpyrrolidone (250-1000 mg/i), citric acid and ascorbic acid (100 mg/l each), thiourea or L-cysteine are also used to prevent oxidation of phenols.
Phloroglucinol, a phenolic compound, is sometimes added to inhibit the enzyme IAA oxidase responsible for the breakdown of IAA.
26.2.12 Gelling agents
Agar, a seaweed derivative, is the most popular solidifying agent. It is a polysaccharide with a high molecular mass and has the capability of gelling media. Solubilized agar forms a gel that can bind water and adsorb compounds. It has been proved that the higher the agar concentration, the stronger the binding of water. Agar is used at a concentration of 0.6 to 1.0% (w/v), although other forms of agar (agarose, phytagar, flow agar, etc.) are also becoming popular. Growth may be adversely affected if the agar concentration is too high. With higher concentrations, the medium becomes hard and does not allow the diffusion of nutrients into the tissues.
Besides agar, the following alternatives are also available.
i. Alginate can be used for plant protoplast culture.
ii. Gelrite at 0.2% can be used for solidification of media. Gelrite gels are remarkably clear in comparison to those formed by agar.
iii. Synthetic polymer biogel P200 (polyacrylamide pellets) or a starch polymer can be used.
26.2.13 pH
pH determines many important aspects of the structure and activity of biological macromolecules. Nutrient medium pH ranges from 5.0 to 6.0 for suitable in vitro growth of explant. pH higher than 7.0 and lower than 4.5 generally stops growth and development. It is observed that the pH before and after autoclaving is different. It generally falls by 0.3 to 0.5 units after autoclaving. If the pH falls appreciably during plant tissue culture (the medium becomes liquid), then a fresh medium should be prepared. It should be known that a starting pH of 6.0 could often fall to 5.5 or even lower during growth. pH higher than 6.0 give a fairly hard medium and a pH below 5.0 does not allow satisfactory gelling of the agar.
26.2.14 Controlled Environment:
Temperature: 25±2 ⁰ C; Humidity: 20 – 98 % RH; Light: 16 h light + 8 h dark are the most usual conditions for plant growth in the laboratory.
26.3 TISSUE CULTURE TECHNIQUES
26.3.1 For Micropropagation
Cell culture and Callus culture
Meristem culture
Shoot tip culture
Node culture /Axillary bud culture
Organ culture
26.3.2 For Crop Improvement
Anther and microspore culture
Somaclonal variation
Embryo / ovule culture
Somatic hybridization ( Protoplast isolation and fusion )
26.4 APPLICATION OF PLANT TISSUE CULTURE:
Since the plant tissue culture work requires costly instruments, technical expertise, and involves a huge capital cost (especially for commercial setup), therefore, it is usually done for a Specific Purpose, which is described as follows :
26.4.1 Crop Improvement / Plant Breeding
Haploid production : For producing homozygous plants, reduction of time involved in conventional breeding. (e.g. Anther / ovule / pollen culture)
Triploid production : For production of seedless fruits (e.g. Endosperm culture)
In vitro pollination and fertilization : To overcome the barrier of sexual in-compatibility between different species or genus of plants in development of distant hybrids.
Zygotic embryo culture : To rescue the embryo after fertilization from abortion
Somatic hybridization and cybridization : To develop inter-generic hybrids, or to develop hybrids between plants that are not crossable with each other
In vitro mutagenesis : Mutagen is applied at cell-level usually with an objective to develop new varieties of crops, with novel traits, through mutagenesis.
Somaclonal and Gametoclonal variant selection : Selection pressure is applied at cell or tissue level and tolerant/ resistant cell types are selected for regeneration. The regenerated plants are expected to be tolerant / resistant to the selection pressure applied. (e.g. resistance to diseases/toxins, higher amino acid content, etc.)
Genetic Transformation: Genes from other organism are used for development of transgenic plants using this technique.
26.4.2 Horticulture / Forestry
Production of disease free plants: For production of disease free plants/ trees. e.g. Virus free plant production (Meristem culture).
1. micropropagation : For mass scale propagation of large number of plants in very short time. Production is independent of season and can continue throughout the year.
26.4.3 General
Cryo-preservation: Long and medium term storage of germ plans
Secondary metabolite production : Production of highly valuable secondary metabolites through in vitro techniques
26.5 BASIC LABORATORY FACILITY AND INFRASTRUCTURE REQUIRED FOR PLAN TISSUE CULTURE WORK:
The following basic laboratory facilities are required for plant tissue culture work
|
Sr. No. |
Infrastructure |
Equipment / Instrumentation facility |
|
i) |
Washing Room |
Washing room with wash basins, bottle washing machine, glassware drying racks. |
|
ii) |
Media Preparation Room |
Stove, pH meter, Autodispenser, weighing scale, refrigerators |
|
iii) |
Sterilization Room |
Autoclave |
|
iv) |
Media Storage Room |
Racks / Shelves for keeping media |
|
v) |
Inoculation Room |
Laminar air flow units, spirit lamps |
|
vi) |
Growth Room/ Culture Room |
Illuminated culture racks, air conditioning system |
|
vii) |
Primary Hardening Facility |
Microprocessor based fully automatic green house, with temperature and humidity control system. |
|
viii) |
Secondary Hardening Facility |
Net House or poly house with fogging system |