Site pages
Current course
Participants
General
Module 1. Design and operational parameters
Module 2. Performance evaluation and maintenance a...
Module 3. Performance evaluation and maintenance a...
Module 4. Performance evaluation and maintenance a...
Module 5. Performance evaluation and maintenance a...
Module 6. Performance evaluation and maintenance a...
Module 7. Biodiesel utilization in CI engines
Lesson 12. Performance evaluation of biogas plants
Biogas plants convert the organic matter into a gaseous fuel. The biogas plants will be designed and constructed based on the feed material availability or the gas production requirement. To assess the performance of any biogas plant the gas production per day has to be monitored. In addition to that the rate of conversion of organic matter into biogas can also be assessed. The energy content of the gas is also decides the energy output from the biogas plant for a given input of feed materials. The procedures to adopt in the assessment of biogas plants are given below.
Gas production
Daily gas production may be measured using gas flow meters. Gas flow meters can be connected with the outlet of the biogas plant. The gas outlet can be observed daily. This can be compared with the actual capacity of the plant.
COD destruction
There is a correlation between Chemical Oxygen Demand (COD) and methane generation in biogas plants. For every kg of destruction of COD, about 0.3 m3 of methane can be generated. Hence, the destruction of COD and generation of biogas in the plant can be assessed by observing the COD content of the influent and effluent and the biogas production.
COD measurement
The Chemical Oxygen Demand (COD) is a measure of oxygen equivalent of that portion of the organic matter in the sample that is susceptible to oxidation by strong chemical oxidant. This is an important and quickly measured parameter for pollution studies of streams and industrial wastewater.
Most of the organic matter is destroyed in boiling mixture of chromic and sulphuric acid. The purpose of running blank is to compensate for any error that may result because of presence of extraneous organic matter in the reagents.
Apparatus
a) COD reflux apparatus of 500 ml flat bottom flask with ground glass joints and condensers.
b) Pipette, burette, measuring cylinders etc.
Reagents Required
a) Standard 0.25 N potassium dichromate (K2Cr2O7) solution
Dissolve 12.259 gm of pure K2Cr2O7 in distilled water and dilute to 1 litre. Add about 120 mgs of sulphuric acid.
b) Sulphuric acid-Silver sulphate reagent
Add 5.5 gms of AgSO4 to 1 Kg of Conc H2SO4. Keep the same overnight.
c) Standard 0.1 N Ferrous Ammonium Sulphate Solution Fe(NH4)2.(SO4)2.6H2O
Dissolve 30 gm of pure salt in distilled water. Add 20 ml of conc H2SO4 and dilute to 1 litre.
d) Ferroin indicator
Dissolve 1.485 gm of 1-10 Phenanthraline monohydrate with 0.695 pure FeSO4.7H2O in distilled water. Dilute to 100 ml. This indicator is commercially available.
e) Con. H2SO4
Procedure
a) Take 0.4 gm of H2SO4 and 20 ml of sample in a reflux flask (if required dilute to suitable degree). Mix well.
b) Add 10 ml of 0.25 N, K2Cr2O7. Drop some pumice stone and slowly add 30 ml of Con H2SO4-AgSO4 reagent.
c) Mix the contents thoroughly and connect the flask to condenser. Reflux for 2 hours.
d) Cool and wash down the condensers. Dilute the mixture to 150 ml by adding distilled water.
e) Add 3 drops of Ferroin indicator and titrate with N/10 Ferrous Ammonium sulphate solution till the colour changes from green to wine red. Note the end point.
f) Perform the same procedure with 'Blank' using distilled water instead of the sample.
Observation table
|
Sl.No |
Sample volume, ml |
ml of Ferrous Ammonium sulphate rundown |
|
|
Sample V1 |
Blank V2 |
||
|
1 |
20 ml |
|
|
|
2 |
20 ml |
|
|
|
3 |
20 ml |
|
|
|
4 |
20 ml |
|
|
(V1-V2) X Normality of Ferrous Ammonium Sulphate X 8000
COD in mgs/litre = ----------------------------------------------------------------------------------
Volume of Sample
If the sample is diluted COD in mgs/litre = COD as above X dilution ratio
(For example if dilution is 10% (i.e. 10 to 100) i.e. 10 ml of sample and 90 ml of distilled water. Then COD in mgs/litre = COD x 10 mg l-1)
Methane content of biogas
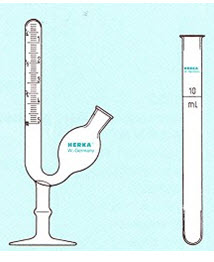
The saccharometer is used to measure the methane content of biogas sample. Saccharometer is filled with 10 per cent NaOH solution. 5 ml of biogas is injected into the saccharometer using syringe. The carbon dioxide present in the biogas will be dissolved in NaOH solution. The methane will be collected on the top of the saccharometer and the volume can be measured.
The constituents of biogas such as methane, carbondioxide etc. can also be measured using gas chromatography method.
Determination of calorific value of biogas
The continuous flow gas calorimeter comprises of a gas flow meter, a gas pressure regulator, a manometer and a combustion chamber with a burner. The gas, whose calorific value has to be determined, is passed through the gas flow meter, then through the regulator and then to the burner. The pressure is measured using the manometer and the temperature is measured at the inlet of the gas meter and also of the products of combustion
The inlet and outlet temperatures of water are measured. The calorimeter is so designed that the gases from the flame pass upwards through the central portion of the calorimeter known as combustion chamber and at the top are deflected down wards and pass through the flues, finally escape into air through the outlet. The calorimeter chamber is cooled by cooling coils. The calorimeter is enclosed in an outer container. The air gap between outer cover and calorimeter acts as insulation against radiation losses. There is an outlet to collect the condensate obtained from the cooling coils as a result of burning of H2 in the gas to water. The condensate is also weighed.
Volume of gas used = V1
Temperature of gas used = T1
Gas manometer reading, = G
Barometric height, = B
Mass of cooling water used = M1
Mass of condensate collected = M2
Cooling water temp. at inlet = Tci
Cooling water temp. at outlet = Tco
Absolute pressure of gas, P1 = [B + (G / 13.6)]
The volume of gas must be converted to the volume, as it would exist at 25oC and 760 mm Hg.
Let this volume be Vo. Then
P 1V1 / T1 = PoVo / To
Vo = (P1/Po) (To / T1 ) V1 =
Now, Energy transferred by gas = Energy gained by the cooling water
Vo x HCV = M1 x (h2- h1)
From steam table,
h2 = Specific enthalpy of water at Tco, J kg-1
h1 = Specific enthalpy of water at Tci, J kg-1
Hence,
HCV = kJ m-3
Latent heat of vaporization = L
Therefore, LCV = HCV - (M2 x L)