Site pages
Current course
Participants
General
Topic 1
Topic 2
Topic 3
Topic 4
Topic 5
Topic 6
Topic 7
Topic 8
Topic 9
Lesson 6. HEAT TREATMENT PROCESSES: HARDENING, TEMPERING, ANNEALING AND NORMALIZING
HEAT TREATMENT PROCESS:HARDENING, TEMPERING, ANNEALING AND NORMALIZING
6.1 Introduction
Steel and other alloys have a large number of applications in engineering practice under varying conditions, requiring different properties in them. At one place they may be subjected to bending while at the other to twisting. They may be required to withstand various types of stresses and as tool materials to have hardness, specially red hardness, combined with toughness along with anon-brittle cutting edge. They may be required to bear static or dynamic loads,revolve at extremely high speeds, operate in highly corrosive media, carry an extremely hard skin with a tough core, subjected to fatigue and creep, etc.Such varying condition of their applications require these materials to possess specific properties of the required order to successfully serve under these conditions. But, a material may lack in some or all of these properties either fully or partially. These deficiencies are fulfilled through the process of heat treatment. Generally all steels can be heat treated as per need. Aluminium is the only non-ferrous metal which can be effectively heat treated.
The process of heat treatment involves heating of solid metals to specified (recrystalisation)temperatures holding them at that temperature and then cooling them at suitable rates in order to enable the metals to acquire the desired properties to the required extents. All this take place because of the changes in size, form,nature and the distribution of different constituents in the micro-structure of these metals. All heat treatment processes, therefore, comprise the following three stages of components:
1. Heating the metal to a predefined temperature.
2. Holding it at that temperature for sufficient time so that the structure of the metal becomes uniform throughout.
3. Cooling the metal at a predetermined rate in a suitable media so as to force the metal to acquire a desired internal structure and thus, obtain the desired properties to the required extent. All this takes place because of the changes in size,form, nature and the distribution of different constituents in the micro-structure of these metals.
6.2 Purpose of Heat Treatment
Metals and alloys are heat treated in order to achieve one or more of the following objectives:
1. To relieve internal stresses set up during other operations like casting, welding, hot and cold working, etc.
2. To improve mechanical properties like hardness,toughness, strength, ductility, etc.
3. To improve machinability
4. To change the internal structure to improve their resistance to heat, wear and corrosion.
5. To effect a change in their grain size.
6. To soften them to make suitable for operations like cold rolling and wire drawing.
7. To improve their electrical and magnetic properties.
8. To make their structure homogenous so as to remove coring and segregation.
9. To drive out trapped gases.
In order to understand the complete mechanism of heat treatment it is essential to know the internal structure, phase transformation, etc. fully. However, a brief review is given:
6.3 Classification of Heat Treatment Processes
Various heat treatment processes can be classified as follows:
1. Annealing.
2. Normalizing.
3. Hardening.
4. Tempering.
5. Case hardening.
6. Surface hardening.
7. Diffusion coating.
6.3.1 Annealing
Annealing is indeed one of the most important heat treatment processes. The internal structure of the metal gets stabilized through this process. This heat treatment is given to the metal so as to achieve one on more of the following objectives:
1. To refine the grains and provide homogenous structure.
2. To relieve internal stresses set up during earlier operations.
3. To soften the metal and, thus, improve its machinability.
4. To effect changes in some mechanical,electrical and magnetic properties.
5. To prepare steel for further treatment or processing.
6. To drive out gases trapped during casting.
7. To produce desired macro structure.
Different type of annealing processes can be classified as follows:
1. Full annealing.
2. Process annealing.
3. Spheroidise annealing.
4. Diffusion annealing.
5. Isothermal annealing.
1. Full annealing
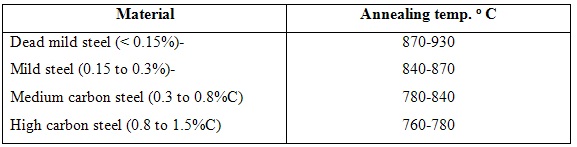
The main objectives of this type of annealing are to soften the metal, relieve its stresses and refine its grain structure. It is also known as high temperature annealing. In this process complete phase recrystallisation takes place and,therefore, all imperfections of the previous structure are wiped out. This involves heating of steel to a temperature about 30o to 50oabove the higher critical point for hypoeutectoid steels, and by the same amount above the lower critical point for hyperuectoid steels, holding it at that temperature for sufficient time to allow the internal changes to take place and then cooling slowly. The steel gets softened by this process,together with an appreciable amount of increase in its ductility and toughness.
Table 6.1 Annealing temperatures for carbon steels
Cooling is done by allowing approximately 3 to 4 minutes time at elevated temperatures per mm thickness of the largest section. High temperature cooling is usually done in the furnace itself by lowering of temperature at the rate of 10 to 30o C below the lower critical temperature. The specimen is then air cooled down to the room temperature. This process makes a course pear litic structure which is quite soft and ductile. An alternate method of cooling after soaking is to embed the metal in a non-conducting material like sand, lime, mica, ash, etc.
2. Process annealing
The purpose of process annealing is to remove the ill effects of cold working and often the metal so that its ductility is restored and it can be again plastically deformed or put to service without any danger of its failure due to fracture. It is also known a slow temperature annealing or sub-critical annealing or commercial annealing.The process is extremely useful for mild steels and low carbon steels and is cheaper and quicker than full annealing. Also, less scale is produced during this process. The main out put of this process is increased ductility and plasticity, improved shock resistance, reduced hardness, improved machinability and removal of internal stresses. During cold working operations like cold-rolling, wire drawing, a metal gets severely strain-hardened . Due to this, the metal is heated to a temperature, generally in the range of 550oC to 650oC, held there for enough time to allow recrystallisation of cold worked metal and,thus, softening to take place and then cooled at a slower rate (normally in air).
3. Spheroidise annealing
The main purpose of spheroidise annealing is to produce a structure of steel which consists of globules or well dispersed spheroids of cementite in ferrite matrix. Following are the main methods through which the above objective can be obtained:
1. High carbon steels: Heating the steel to a temperature slightly above the lower critical point (say between 730oC to 770oC,depending upon the carbon percentage), holding it at that temperature for sufficient time and than cooling it in the furnace to a temperature 600oCto 550oC, followed by slowly cooling it down to room temperature instill air.
2. Tool steels and high-alloy steels: Heating to a temperature of 750oC to 800oC, or even higher, holding at that temperature for several hours and then cooling slowly.
4. Diffusion annealing
The purpose of diffusion annealing is to remove the heterogeneity in the chemical composition of steel ingots and heavy castings This process is mainly used before applying full annealing to steel castings. In this process , the metal is heated to a temperature between 1100oC to 1200oC,where diffusion occurs and grains are homogenized. The metal piece being treated is held at the diffusion temperature for a short time to allow complete diffusion and than cooled down to between 800oC to 850oC by keeping it inside the shut off furnace for a period of about 6 to 8 hours. Then it is removed from the furnace and cooled in air down to the room temperature. Then full annealing is performed.
5. Isothermal annealing
The isothermal annealing consists of heating steel to austenite state and then cooling it down to a temperature of about 630oC to 680oCat a relatively faster rate. It is followed by holding it at this constant temperature (i.e isothermal) for some time and then cooling it down to the room temperature at a rapid rate. During the isothermal holding full decomposition to pearlite structure takes place and that is why the process is known as isothermal annealing. Because of the two rapid coolings the total annealing time is considerably reduced.
6.3.2 Normalizing
The normalizing process is similar to annealing in sequence but vary in the heating temperature range, holding time and the rate of cooling. Heating temperature of steel is 40oC to 50oCabove the higher critical point, held at that temperature for a relatively very short period of time (about 15 min.) and then cooled down to room temperature in still air. This heat treatment is commonly used as the final heat treatment for such articles which are supposed to be subjected to higher stress during operation. Due to this treatment internal stress caused during previous operations are removed, internal structure is refined to fine grains and mechanical properties of steel are improved. This process also improves the impact strength, yield point and ultimate tensile strength of steels. As compared to the annealed steels of the same composition the normalized steels will be less ductile but stronger and harder. For improvement of the mechanical properties normalizing process should be preferred and to attain better machinability, softening and greater removal of internal stress annealing process should be employed.
6.3.3 Hardening
This process is widely applied to all cutting tools, all machine parts made from alloy steels, dies and some selected machine parts subjected to heavy duty work. In hardening process steel is heated to a temperature within the hardening range, which is 30oC to 50oC above the higher critical point for hypoeutectoid steels and by the same amount above the lower critical point for hypoeutectoid steels, holding it at that temperature for sufficient time to allow it to attain austenitic structure and cooled rapidly by quenching in a suitable medium like water, oil or salt both.
In the process of hardening the steel is developed in such controlled conditions,by rapid quenching, that the transformation is disallowed at the lower critical point and by doing so we force the change to take place at a much lower temperature. By rapid cooling the time allowed to the metal is too short and hence transformation is not able to occur at the lower critical temperature.
6.3.4 Tempering
A hardened steel piece, due to martensitic structure, is extremely hard and brittle, due to which it is found unsuitable for most practical purposes. So a subsequent treatment is required to obtain a desired degree of toughness at the cost of some strength and hardness to make it suitable for use. It is especially true in case of the tools. This is exactly what is mainly aimed at through tempering of steel. This process enables transformation of some martensite into ferrite and cementite. The exact amount of martensite transformed into ferrite plus cementite will depend upon the temperature to which the metal is reheated and the time allowed for the transformation.
The process involves reheating the hardened steel to a temperature below the lower critical temperature, holding it at that temperature for sufficient time and then cooling it slowly down to the room temperature.
When the hardened steel is reheated to a temperature between 100oC to 200oCsome of the interstitial carbon is precipitated out from martensite to form acarbide called epsilon carbide. This leads to the restoration of BCC structure in the matrix. Further heating to between 200oC 400oCenables the structure to transform to ferrite plus cementite. Further heating to between 400oC and 550oC leads to the nucleation and growth of a new ferrite structure, rendering the metal weaker but more ductile.If steel is heated above 550oC the cementite becomes spheroidised,and if heating is continued even beyond the structure will revert back to the stable martensite. As such, if a good impact strength is desired reheating should not extend beyond 300o to 350oC. The section thickness of the components being treated also have a decisive effect on the results. Heavy components and thicker sections required longer tempering times then the lighter and thinner ones.
6.3.4.1 Types of tempering
On the basis of the ranges of temperatures to which the components are reheated for tempering, the tempering procedures are classified as follows:
1. Low temperature tempering
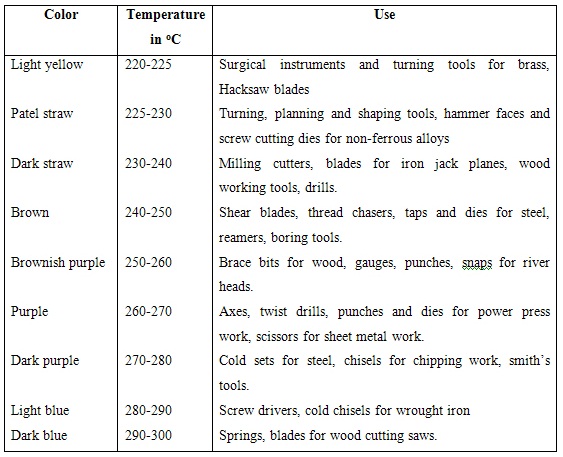
This treatment results in reduction of internal stresses and improvement in toughness and ductility without any appreciable loss in hardness. The heating range for this type of tempering is from 150oC to 250oC. The different colours appearing on the surface of the metal are indicative of the approximate temperature attained by it. Carbon tool steels, low alloy tool steels, case carburized and surface hardened parts, measuring tools, etc are tempered by this method. Approximate temperatures, corresponding colours and the tools for whose tempering they are used are given in following table.
Table 6.2 Approximate tempering temperatures and temper colours for tools
2. Medium temperature tempering
This process involves reheating the component to a temperature range between 350oCto 450oC, holding at that temperature for sufficient time and then cooling it to room temperature. This method of tempering is used to increase the toughness of steel but reduces the hardness. It also increases the ductility and decresess the strength. It is mainly used for articles where a high yield strength, coupled with toughness, is a major requirement and subjected to impact loading, like coils and springs, hammers, chisels, etc.
3. High temperature tempering
The process involves reheating the hardened steel to a temperature between 500oC to 650 o, holding it there for a certain time and then cooling it down to the room temperature.This process enables the steel attaining high ductility while retaining enough hardness. This provides a micro-structure which carries a useful combination of good strength and toughness with complete elimination of internal stresses .E.g.Crankshafts, connecting rods and gears
6.3.5 Tempering bath
Mainly following three types of tempering baths are used for tempering of steel parts and cutting tools:
Lead bath : Lead or lead alloy bath may be used for tempering steel parts. The parts are preheated and then immersed in the bath, which is already heated to the tempering temperature. Once the parts reach the tempesing temperature they are taken out and cooled to attain the required temper.
Oil bath: Oil baths can be employed for various temperature ranges. Mineral oils are commonly used for these baths. Light oil baths are used for temperatures upto 230 oC only. Heavy oil baths can be used for heating range from343 oC to 370 oC. For oil heating the bath temperature is first raised to the required tempering range and then partially heated component is immersed in it. If the temperature of the bath falls below the required level both the bath and the immersed component can be heated together to the tempering temperature. After the component has reached the required temperature it is removed and immersed in a tank of caustic soda, followed by quenching in a hot water bath.