Site pages
Current course
Participants
General
Module 1. Moisture content and its determination.
Module 2. EMC
Module 3. Drying Theory and Mechanism of drying
Module 4. Air pressure within the grain bed, Shred...
Module 6. Study of different types of dryers- perf...
Module 5. Different methods of drying including pu...
Module 7. Study of drying and dehydration of agric...
Module 8. Types and causes of spoilage in storage.
Module 9. Storage of perishable products, function...
Module 10. Calculation of refrigeration load.
Module 11. Conditions for modified atmospheric sto...
Module 12. Storage of grains: destructive agents, ...
Module 13. Storage of cereal grains and their prod...
Module 14. Storage condition for various fruits an...
Module 15. Economics aspect of storage
Lesson.18 High Temperature Drying
One of the ways to shorten the drying time is to supply heat by infrared radiation. This method of heating is especially suitable to dry thin layers of material with large surface exposed to radiation. Infrared radiation is transmitted through water at short wavelength, while at long wavelength; it is absorbed on the surface [45]. Hence, drying of thin layers seems to be more efficient at far-infrared radiation-FIR (25–100 Am), while drying of thicker bodies should give better results at near-infrared radiation-NIR (0.75–3.00 Am) [46]. Sharma et al.[46] dried onion slices at infrared power levels 300, 400 and 500 W, drying air temperatures of 35°, 40° and 45 °C and inlet drying air velocities 1.0, 1.25 and 1.5 m/s. They reported that drying time reduced by about 2.25 times on increasing infrared power from 300 to 500 W, air temperature 35° to 45 °C and air velocity from 1.0 to 1.5 m/s. Effective moisture diffusivity was significantly influenced by infrared power and air temperature. Baysal et al. [47] dehydrated carrots in a tray drier at 70 °C with 0.86 m/s air velocity, in microwave oven at power density of 6 W/g (60 s power on and power off for 15 s) and by infrared drying at different time temperature combinations of 105°C for 15 min, 100°C for 30 min and 95°C for 40 min. The Infrared dehydrated carrot had the best rehydration capacity.
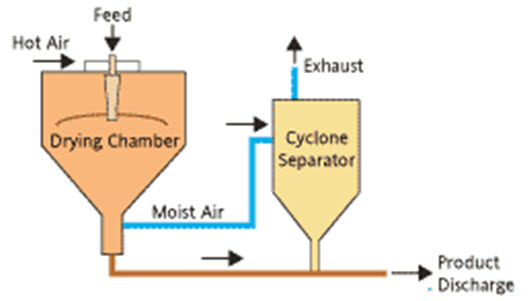
Spray Drying
Spray drying has important application in functional food formulation. The spray drying process consists of the conversion of a spray of pumpable liquid (i.e., juices, slurries, and purees) into a dry particulate (i.e., powder, granules, or agglomerate) by exposure to a hot (150 to 200°C) medium (Sunjka et al., 2008). Operating and dryer components that influence the final product include the feed rate, temperature of the inlet drying air, pressure of compressed air at the nozzle, air flow (i.e., cocurrent, counter current, or mixed flow), atomizer design, and air heating method. Spray dryers are the most widely used drying systems for the formation of powdered food additives and flavors in the dairy, beverage, and pharmaceutical industries. This technique enables the transformation of feed from a fluid state into dried particulate form by spraying the feed into a hot drying medium. It is a continuous particle processing drying operation. The feed can be a solution, suspension, dispersion or emulsion. The dried product can be in the form of powders, granules or agglomerates depending upon the physical and chemical properties of the feed, the dryer design and final powder properties desired (Michael, 1993).
Spray drying process mainly involves five steps:
-
Concentration: feedstock is normally concentrated prior to introduction into the spray dryer.
-
Atomization: the atomization stage creates the optimum condition for evaporation to a dried product having the desired characteristics.
-
Droplet-air contact: in the chamber, atomized liquid is brought into contact with hot gas, resulting in the evaporation of 95%+ of the water contained in the droplets in a matter of a few seconds.
-
Droplet drying: moisture evaporation takes place in two stages- 1) during the first stage, there is sufficient moisture in the drop to replace the liquid evaporated at the surface and evaporation takes place at a relatively constant rate (Keey & Pham, 1976), and 2) the second stage begins when there is no longer enough moisture to maintain saturated conditions at the droplet surface, causing a
-
dried shell to form at the surface. Evaporation then depends on the diffusion of moisture through the shell, which is increasing in thickness.
-
Separation: cyclones, bag filters, and electrostatic precipitators may be used for the final separation stage. Wet Scrubbers are often used to purify and cool the air so that it can be released to atmosphere.