Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Module 11. Lubricants properties, mechanism, class...
Module 12. Polymers type of polymerization, proper...
Lesson 10. Disadvantages of hard Water
10.0 DISADVANTAGES OF HARD WATER
10.1.1 In domestic use:
(i) Washing: Hard water, when used for washing purposes, does not lather freely with soap. On the other hand, it produces sticky precipitates of calcium and magnesium soaps. The formation of such insoluble, sticky precipitated continues, till all calcium and magnesium salts present in water are precipitated. After that, the soap (e.g., sodium stearate) gives lather with water. Thus;
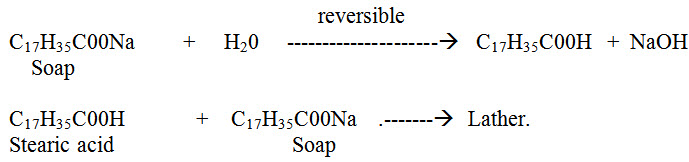
This causes wastage of soap being used. Moreover, the sticky precipitate (of calcium and magnesium soaps) adheres on the fabric/cloth giving spots and streaks. Also presence of iron salts may cause staining of cloth.
(ii) Bathing: Hard water does not lather freely with soap solution, but produces sticky scum on the bath-tub and body. Thus, the cleansing quality of soap is depressed and a lot of it is wasted.
(iii) Cooking: Due to the presence of dissolved hardness-producing salts, the boiling point of water is elevated. Consequently, more fuel and time are required for cooking certain foods such as pulses, beans and peas do not cook soft in hard water. Also tea or coffee, prepared in hard water, has an unpleasant taste and muddy-looking extract. Moreover, the dissolved salts are deposited as carbonates on the inner walls of the water heating utensils.
(iv)Drinking: Hard water causes bad effect on our digestive system. Moreover, the possibility of forming calcium oxalate crystals in urinary tracks is increased.
10.1.2 Industrial use: (i) Textile industry: Hard water causes much of the soap (used in washing yarn, fabric etc.) to go as waste, because hard water cannot produce good quality of lather. Moreover, precipitated of calcium and magnesium soaps adhere to the fabrics. These fabrics, when dyed latter on, do not produce exact shades of color. Iron and manganese salts-containing water may cause colored spots on fabrics, thereby spoiling their beauty.
(ii) Sugar industry: Water containing sulphates, nitrates, alkali carbonated, etc., if used in sugar refining, causes difficulties in the crystallization of sugar. Moreover, the sugar so-produced may be deliquescent.
(iii) Dyeing industry: The dissolved calcium, magnesium and iron salts in hard water may react with costly dyes, forming undesirable precipitated, which yields impure shades and give spots on the fabrics being dyed.
(iv) Paper industry: Calcium and magnesium salts tend to react with chemicals and other materials employed to provide a smooth and glossy (i.e., shining) finish to paper. Moreover, iron salts may even affect the color of the paper being produced.
(v) Laundry: Hard water, if used in laundry, causes much of the soap used in washing to go as waste. Iron salts may even cause coloration of the clothes.
(vi) Concrete making: Water containing chlorides and sulphates, if used for concrete making, affects the hydration of cement and the final strength of the hardened concrete.
(vii) Pharmaceutical industry: Hard water, if used for preparing pharmaceutical products (like drugs, injections, ointments, etc.) may produce certain undesirable products in them.
10.1.3 In steam generation in boilers: For steam generation, boilers are almost invariably employed. If the hard water is fed directly to the boilers, there arise many troubles such as: (i) scale and sludge formation, (ii) corrosion,(iii) priming and foaming and (iv) caustic embrittlement.
10.2 SOFTENING METHODS
Water used for industrial purposes (such as for steam generation) should be sufficiently pure. It should, therefore, be freed from hardness-producing salts before put to use. The process of removing hardness-producing salts from water, is known as softening of water. In industry, main three methods employed for water softening. Like;
10.2.1 Lime-soda process: In this method, the soluble calcium and magnesium salts in water are chemically converted into insoluble compounds, by adding calculated amounts of lime (Ca(OH)2) and soda (Na2CO3). Calcium carbonate (CaCO3) and Magnesium hydroxide (Mg(OH)2) are precipitated and are filtered off.
10.2.1.1 Cold lime-soda process: In this method, calculated quantity of chemical (lime and soda) is mixed with water at room temperature. At room temperature, the precipitates formed are finally divided, so they do not settle down easily and con not be filtered easily. It is essential to add small amount of coagulants which hydrolyze to flocculent, gelatinous precipitate of aluminum hydroxide, and entraps the fine precipitates. Use of sodium aluminates as coagulant also helps the removal of silica as well as oil, if present in water. Cold L-S process provides water, containing a residual hardness of 50 to 60 ppm.
Method: Raw water and calculated quantities of chemicals (lime + soda + coagulant) are fed from the top into the inner vertical circular chamber, fitted with a vertical rotating shaft carrying a number of paddles. As the raw water and chemicals flow down, there is a vigorous stirring and continuous mixing, whereby softening of water takes place. As the softened water comes into the outer co-axial chamber, it rises upwards. The heavy sludge (or precipitated flock settles down in the outer chamber by the time the softened water reaches up. The softened water then passes through a filtering media (usually made of wood fibers) to ensure complete removal of sludge. Filtered soft water finally flows out continuously through the outlet at the top. Sludge settling at the bottom of the outer chamber is drawn off occasionally.
10.2.1.2 Hot lime-soda process involves in treating water with softening chemicals at a temperature of 80 to 150 0C. Since hot process is operated at a temperature close to the boiling point of the solution, so :
(i) the reaction proceeds faster.
(ii) the softening capacity of hot process is increased to many fold.
(iii) the precipitate and sludge formed settle down rapidly and hence, no coagulants are needed.
(iv) much of the dissolved gases (such as CO2 and air) driven out of the water
(v) viscosity of softened water is lower, so filtration of water becomes much easier.
This in-turn increases the filtering capacity of filters.
(vi) hot lime-soda process produces water of comparatively lower residual hardness of 15 to 30 ppm.
Advantages of Lime-Soda process :
(i) it is very economical.
(ii) if this process is combined with sedimentation with coagulation, lesser amounts of coagulants shall be needed.
(iii) The process increases the pH value of the treated-water; thereby corrosion of the distribution pipes is reduced.
(iv) Besides the removal of hardness, the quantity of minerals in the water is reduced.
(v) To certain extent, iron and manganese are also removed from the water.
(vi) Due to alkaline nature of treated-water, amount of pathogenic bacteria in water is considerably reduced.
Disadvantage of Lime-Soda process:
(i) For efficient and economical softening, careful operation and skilled supervision in required.
(ii) Disposal of large amounts of sludge (insoluble precipitate) poses a problem. However, the sludge may be disposed off in raising low-lying areas of the city.
(iii) This can remove hardness only up to 15 ppm, which is not good for boilers.
10.2.2 Zeolite or pemutits process : Chemical structure of sodium zeolite may be represented as : Na2O.Al203.xSiO2.yH2O where x = 2-10 and y = 2-6. Zeolite is hydrated sodium alumino silicate, capable of exchanging reversibly its sodium ions for hardness-producing ions in water. Zeolites are also known as permutits. Zeolites are of typo types : (a) Natural zeolites are non-porous. For example, natrolite, Na2O.Al3OS4SiO2.2H2O. (b) Synthetic zeolites are porous and possess gel structure. They are prepared by heating together china clay, feldspar and soda ash. Such zeolites possess higher exchange capacity per unit weight than natural zeolites.
Process: For softening of water by zeolite process, hard water is percolated at a specified rate through a bed of zeolite, kept in a cylinder. The hardness causing ions (Ca2+, Mg2+, etc.) are retained by the zeolite as CaZe and MgZe; while the outgoing water contains sodium salts. Reactions taking place during the softening process are;

Regeneration: After some time, the zeolite is completely converted into calcium and magnesium zeolites and it stops to soften water, i.e., it gets exhausted. At this stage, the supply of hard water is stopped and the exhausted zeolite is reclaimed by treating the bed with a concentrated (10%) brine (NaCl) solution.
CaZe or MgZe + 2NaCI -----> Na2Ze + CaCI2 or MgCl2
The washings containing CaCl2 and MgCl2 are led to drain and the regenerated zeolite bed thus-obtained is used again for softening purpose.
Limitation of zeolite process:
(i) if the supply of water is turbid, the suspended matte must be removed (by coagulation, filtration, etc.), before the water is admitted to the zeolite bed; otherwise the turbidity will clog the pores of zeolite bed, thereby making it inactive.
(ii) if water contains large quantities of coloured ions such as Mn2+ and Fe2+, they must be removed first, because these ions produce manganese and iron zeolites, which cannot be easily regenerated.
(iii) Mineral acids, if present in water, destroy the zeolite bed and, therefore, they must be neutralized with soda, before admitting the water to the zeolite softening plant.
Advantages of zeolite process:
(i) it removes the hardness almost completely and water of about l0 ppm hardness is produced.
(ii) The equipment used is compact, occupying a small space.
(iii) No impurities are precipitated, so there is no danger of sludge formation in the treated-water at a later stage.
(iv) The process automatically adjusts itself for variation in hardness of incoming water.
(v) it is quite clean.
(vi) it requires less time for softening.
(vii) it requires less skill for maintenance as well as operation.
Disadvantages of zeolite process:
(i) The treated-water contains more sodium salts than in lime-soda process.
(ii) The method only replaces Ca2+ and Mg2+ ions by Na+ ions, but leaves all the acidic ions like HCO3- and CO32- as such in the softened water. When such softened water (containing NaHCO3, Na2CO3 etc.) is used in boilers for steam generation, sodium bicarbonate decomposes producing CO2, which causes corrosion ; and sodium carbonate hydrolysis to sodium hydroxide, which causes caustic embrittlement.
(iii) High turbidity water cannot be treated efficiently by this method, because fine impurities get deposited on the zeolite bed, thereby creating problem for its working.
10.2.3 Ion exchange or de-ionization or de-mineralization process : Ion-exchange resins are insoluble, cross-linked, long chain organic polymers with a microporous structure, and the “functional groups" attached to the chains are responsible for the ion-exchanging properties. Resins containing acidic functional groups (− C00H, − SO3H etc.) are capable of exchanging their H+ ions with other cations, which comes in their contact; whereas those containing basic functional groups (− NH2 = NH as hydrochloride salt) are capable of exchanging their anions with other anions, which comes in their contact. The ion-exchange resins may be classified as:
(i) Cation exchange resins (RH+) are mainly styrene-divinyl benzene copolymers, which on sulphonation or carboxylation, become capable to exchange their hydrogen ions with the cations in the water.
(ii) Anion exchange resins (R'OH-) are styrene-divinyl benzene or amine formaldehyde copolymers, which contain amino or' quaternary ammonium or quaternary phosphonium or tertiary sulphonium groups as an integral part of the resin matrix. These, after treatment with dil. NaOH solution, become capable to exchange their OH- anions with anions in water.
Process: The hard water is passed first through cation exchange column, which removes all the cations like Ca2+, Mg2+, etc. from it, and equivalent amount of H+ ions are released from this column to water. Thus;
2RH+ + Ca2+ --------.> R2Ca2+ + 2H+
2RH+ + Mg2+ ------.> R2Mg2+ + 2H+
After cation exchange column, the hard water is passed through anion exchange column, which removes all the anions like SO42−, Cl−, etc. present in the water and equivalent amount of OH− ions are released from this column to water. Thus:
R'OH- + Cl- ---..------.> R'Cl- + OH-
2R'OH- + SO42- ---..--> R'2SO42- + 2OH-
2R'OH- + CO32- ----.-> R'2CO32- + 2OH-
H+ and OH- ions (released from cation exchange and anion exchange columns respectively) get combined to produce water molecule.
H+ + OH- --> H2O
Thus, the water coming out from the exchanger is free from cations as well as anions. Ion-free water, is known as deionized or demineralised water.
Regeneration: When capacities of cation and anion exchangers to exchange H+ and OH- ions respectively are lost, they are then said to be exhausted. The exhausted cation exchange column is regenerated by passing a solution of dil. HCl or dil H2SO4. The regeneration can be represented as :
R2Ca2+ + 2H+ ----> 2RH+ + Ca2+ (washing)
The column is washed with deionised water and washing (which contains Ca2+, Mg2+, etc. and Cl- or SO42- ions) is passed to sink or drain The exhausted anion exchange column is regenerated by passing a solution of dil. NaOH. The regeneration can be represented as :
R'2S042- + 2OH- ---> 2R'OH- + SO42-(washing)
The column is washed with deionised water and washing (which contains Na+ and SO42- or Cl- ions) is passed to silk or drain. The regenerated ion exchange resins are then used again.
Advantages:
(i) The process can be used to soften highly acidic or alkaline waters.
(ii) It produces water of very low hardness (say 2 ppm). So it is very good for treating water for use in high-pressure boilers.
Disadvantages:
(i) The equipment is costly and more expensive chemicals are needed.
(ii) If water contains turbidity, then the output of the process is reduced. The turbidity must be below 10 ppm. If it is more, it has to be removed first by coagulation and then by filtration.