Site pages
Current course
Participants
General
Module- 1 Engineering Properties of Biological Mat...
Module- 2 Physical Properties of Biomaterials
Module- 3 Engineering Properties
Module- 4 Rheological Properties of Biomaterials
Module- 5 Food Quality
Module- 6 Food Sampling
Module- 7 Sensory quality
Module 8. Quality Control and Management
Module 9. Food Laws
Module 10. Standards and regulations in food quali...
Lesson 32. Sanitation in food industry
Lesson 18. Flavor extraction and Measurement methods
Extraction of flavors:
I. Extraction Techniques
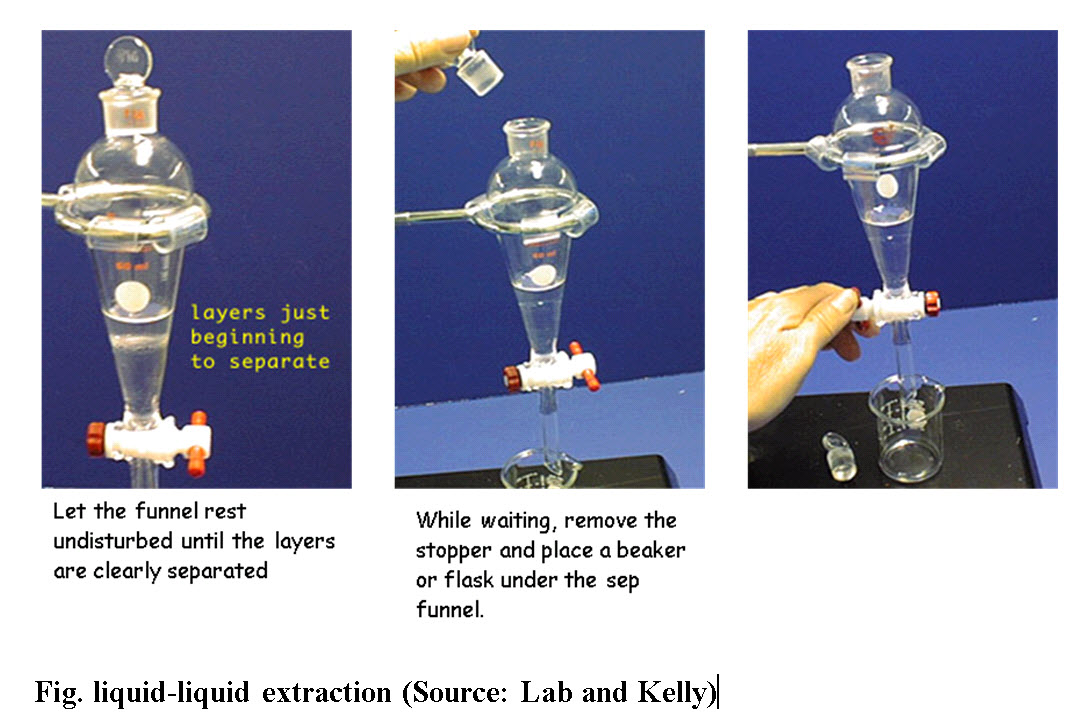
A. Solvent Extraction: Solvent extraction is a selective separation procedure for isolating and concentrating a valuable material from an aqueous solution with the aid of organic acids. The extraction process depends on the solubility of the flavouring compounds in the solvent. A compound can be separated from impurities in a solution by extracting the compound from the original (or first) solvent into a second solvent. For the process to be selective, the compound must be more soluble in the second solvent than in the first solvent, and the impurities must be insoluble in the second solvent. Additionally, the two selected solvents must be immiscible, or not soluble in one another, so that they produce two separate solvent layers. After dissolving the mixture in the first solvent, the solution is added to a second solvent.
B. Solid Phase Extraction (SPE): The principle of SPE is similar to that of liquid-liquid extraction (LLE), involving a partitioning of solutes between two phases. However, instead of two immiscible liquid phases, as in LLE, SPE involves partitioning between a liquid (sample matrix or solvent with analytes) and a solid (sorbent) phase. SPE is typically performed by loading the complex sample onto a preconditioned extraction cartridge containing a chromatographic sorbent.
C. Solid phase micro extraction (SPME): solid-phase microextraction is a solvent-free sampling technique based on the sorption characteristics (adsorption or absorption) of fiber coating materials. The analytes (volatiles or semivolatiles) from gaseous, liquid, or solid matrices are first released from the matrices and sorbed onto a fiber coated with an ad(ab)sorbent polymer introduced into the headspace. Following sorption, analytes are either thermally desorbed onto a gas chromatographic (GC) inlet or solvent desorbed into a high-performance liquid chromatographic (HPLC) inlet.
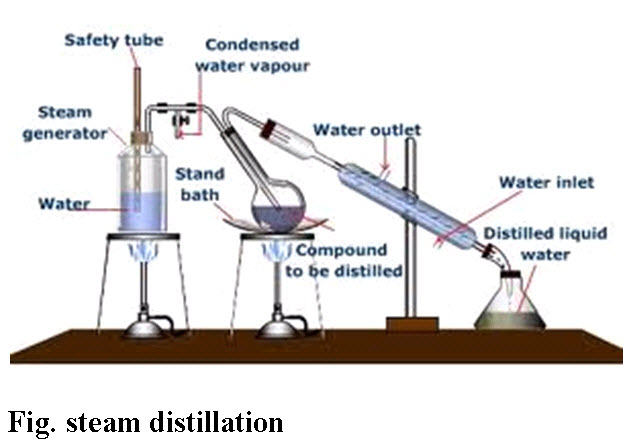
D. Steam Distillation: Many organic compounds tend to decompose at high sustained temperatures. Separation by normal distillation would then not be an option, so water or steam is introduced into the distillation apparatus. By adding water or steam, the boiling points of the compounds are depressed, allowing them to evaporate at lower temperatures, preferably below the temperatures at which the deterioration of the material becomes appreciable. If the substances to be distilled are very sensitive to heat, steam distillation can also be combined with vacuum distillation. After distillation the vapors are condensed as usual, usually yielding a two-phase system of water and the organic compounds, allowing for decantation.
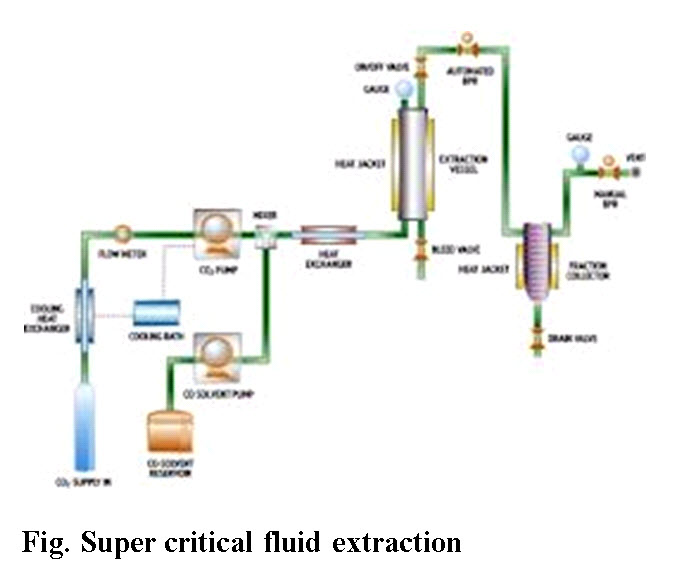
E. Super critical fluid extraction: A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid. In addition, close to the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be altered. Supercritical fluids are suitable as a substitute for organic solvents in a range of industrial and laboratory processes. Carbon dioxide and water are the most commonly used supercritical fluids.
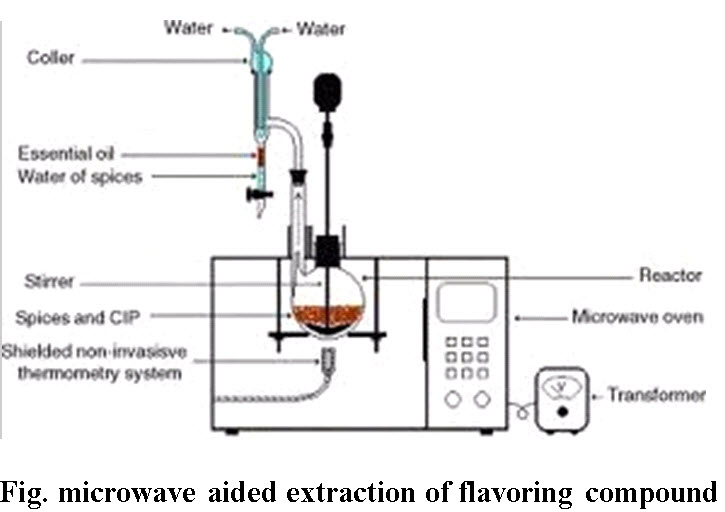
F. Microwave aided extraction: Microwaves cause bipolar rotation of polar molecules like water, which generates heat due to molecular friction. Even though dried plant material is used for extraction in most cases, but still plant cells contain minute microscopic traces of moisture that serves as the target for microwave heating. The moisture when heated up inside the plant cell due to microwave effect, evaporates and generates tremendous pressure on the cell wall due to swelling of the plant cell. The pressure pushes the cell wall from inside, stretching and ultimately rupturing it, which facilitates leaching out of the active constituents from the ruptures cells. This process is very fast compared to other techniques and saves considerable amount of solvent and time.
G. Ultrasound aided extraction: Sound waves can be classified into three categories i.e. supersonic (frequency< 20 Hz), audible (20 Hz <frequency>20 kHz), or ultrasound (frequency>20 kHz). Ultrasound is simply sound pitched above human hearing. Ultrasound waves can be classified into two categories(i) high-power, low-frequency ultrasound from 20 kHz to 2 MHz, (ii)) and 5 MHz to several GHz), low-power ultrasound. Power ultrasound usually refers to the frequency range between 20-40 kHz. Power ultrasound has been used to accelerate processes such as dehydration, drying, freezing and thawing, tenderization of meat, crystallization of lactose and fat and to improve processes such as cutting, extraction, emulsification, ageing of wines and esterification etc. Power ultrasound waves, when propagated through any food medium, result in cavitations and bubble formation, compression and rarefaction, cavitations, heating due to absorbance of acoustic energy on the product interfaces and streaming. Cavitation occurs when acoustic waves propagate through liquid media, forming very small bubbles. If the bubbles are of critical size, as determined by the wave frequency; then they explode releasing energy in the form of impulses which can have local point temperature about 1000K and pressure of 1000 atm. But it does not raise the temperature of the liquid food material; if the bubbles explode near the boundary then it gets evaporated. These phenomena result in structural disintegration and energy dissipation to the medium. Structural disintegration leading to cell permeabilization makes way for the water molecules to travel to the surface and this phenomena aid in mass transfer rate during extraction process.
II. Headspace Sampling
A. Static Headspace
B. Dynamic Headspace
C. Thermal Desorption
Flavor analysis: Flavor may be evaluated with either instrumental or sensory methods, but most scientists would agree that sensory methods are the most critical to this particular quality attribute. Instrumental techniques may determine that tens or hundreds of compounds are present in a particular food product, but such methods do not give a measure of the contribution of that specific compound unless they are accompanied by a sensory measurement of odor or flavor activity. For this reason, flavor may be the most challenging quality attribute to both measure and correlate to consumer acceptability. There are some characteristics of flavor that may be determined instrumentally.
Sensory Analysis
- Discrimination tests
- Difference tests
- Threshold tests
- Analytical intensity rating testsConsumer tests
- Types of scales
- Descriptive analysis
- Time-intensity
- Consumer Tests
- Instrumental Methods
Volatile analysis:
-
GC-MS
-
GC-OlfactometryElectronic nose
-
Flavor Identification by Spectrometric Methods
-
Ultra Violet Spectrometry
-
Infrared Spectrometry
-
Nuclear Magnetic Resonance Spectrometry
-
Mass Spectrometry
-
-
- Electronic Nose
Sweetness can be approximated by HPLC determination of individual sugars, or more rapidly but less accurately by a refractometer or hydrometer that measures total soluble solids. Indicator papers exist for rapid determination of glucose in some commodities, such as potatoes. It is possible to measure chloride and/or sodium content as an approximation of saltiness. Sourness may be determined by either pH or more accurately by measurement of total acidity. Both indicator papers and pH meters are available for the determination of pH. Astringency may be indicated by measuring total phenolics and bitterness by analysis of compounds such as alkaloids or glucosides.
Some terms in flavor:
1) Seasoning: An item added to enhance the natural flavors of a food without changing its taste, eg. Salt is the most common seasoning.
2) Flavoring: An item that adds a new taste to food and alters its natural flavors, eg. Herbs (Any of a large group of aromatic plants whose leaves, stems or flowers are used as a flavoring, used either dry or fresh), spices (Any of a large group of aromatic plants whose bark, roots, seeds, buds or berries are used as flavoring. Usually used in dry form, whole or ground), vinegars and condiments (Any item added to a dish for flavor, including herbs, spices and vinegars. Also refers to cooked or prepared flavorings such as prepared mustards, relishes, bottled sauces and pickles).
Reference:
Barrett, D. M., Beaulieu, J.C. and Shewfelt, R. (2010). Color, Flavor, Texture, and Nutritional Quality of Fresh-Cut Fruits and Vegetables: Desirable Levels, Instrumental and Sensory Measurement, and the Effects of Processing, Critical Reviews in Food Science and Nutrition, 50: 5, 369-389.
Voilley A. and Etievant P. (ed). 2006. Flavour in food. Woodhead Publishing Limited, Cambridge, England.
Da-Mi Jung, Susan E. Ebeler (2003) . Headspace Solid-Phase Microextraction
Method for the Study of the Volatility of Selected Flavor Compounds. J. Agric. Food Chem., 51, 200-205