Site pages
Current course
Participants
General
Module- 1 Engineering Properties of Biological Mat...
Module- 2 Physical Properties of Biomaterials
Module- 3 Engineering Properties
Module- 4 Rheological Properties of Biomaterials
Module- 5 Food Quality
Module- 6 Food Sampling
Module- 7 Sensory quality
Module 8. Quality Control and Management
Module 9. Food Laws
Module 10. Standards and regulations in food quali...
Lesson 32. Sanitation in food industry
Lesson 29. HACCP
29.1 Introduction:
HACCP stands for Hazard Analysis and Critical Control Point. Hazard is a biological, chemical or physical agent that is reasonably likely to cause illness or injury in the absence of its control. Hazards can be harmful microorganisms or chemical and/or physical contaminants. To ensure safe food, HACCP system is designed to identify hazards, establish controls and monitor these controls. HACCP is a preventive system of hazard control rather than a reactive one. Food processors can use it to ensure safer food products for consumers..
The Pillsbury Co. pioneered the application of the HACCP concept to food production during its efforts to supply food for the U.S. space program in the early 1960s.. It is not a zero-risk system, but it is designed to minimize the risk of food-safety hazards. In an assessment of the effectiveness of food regulation in the United States, the National Academy of Sciences (NAS) recommended in 1985 that the HACCP approach be adopted by all regulatory agencies and that it be mandatory for food processors Since then globally this system has been adopted to ensure safety of foods.
HACCP is a preventive system for ensuring food safety, but it is not a stand-alone system. HACCP must be built upon current food-safety programs such as Good Manufacturing Practices (GMPs) and others to make it work.
29.2 HACCP Plan:
To perform a hazard analysis for the development of a HACCP plan, food processors must gain a working knowledge of potential hazards. The HACCP plan is designed to control all reasonably likely food-safety hazards. Such hazards are categorized into three classes: biological, chemical and physical.
Biological Hazards
These hazards can come from raw materials or from food-processing steps used to make the final product. Microorganisms live everywhere: air, dirt, fresh and salt water, skin, hair, animal fur and plants. Microorganisms are classified into various groups. A few groups important in foods include yeasts, molds, bacteria, viruses and protozoa. Although thousands of kinds of microorganisms exist, only a few pose hazards to humans. Without adequate food, water and temperature, microorganisms stop growing and multiplying. Some die and others stop functioning until they get the elements they need. Some preservation methods, such as drying or smoking, control of water or nutrients in food, make these essential elements unavailable to microorganisms.
Chemical Hazards
Chemical contamination can happen at any stage in food production and processing. Chemicals can be helpful and are purposefully used with some foods, such as preservatives. The presence of a chemical may not always represent a hazard. The amount of the chemical may determine whether it is a hazard or not. Some may require exposure over prolonged periods to have a toxic effect. Regulatory limits are set for some of those contaminants.
Chemical hazards can be separated into three categories:
Naturally occurring chemicals.
Intentionally added chemicals.
Unintentionally or incidentally added chemicals.
Physical Hazards
Physical hazards include any potentially harmful extraneous matter not normally found in food. When a consumer mistakenly eats the foreign material or object, it is likely to cause choking, injury or other adverse health effects. The source of the hazard is often easy to identify.
29.3 Guidelines for the Application of the HACCP System
Prior to application of HACCP to any sector of the food chain, that sector should be operating according to the Codex General Principles of Food Hygiene, the appropriate Codex Codes of Practice and appropriate food safety legislation like FSSAI. Management commitment is necessary for implementation of an effective HACCP system.
During hazard identification, evaluation and subsequent operations in designing and applying HACCP systems, consideration must be given to the impact of raw materials, ingredients, food manufacturing practices, role of manufacturing processes to control hazards, likely end-use of the product, categories of consumers of concern, and epidemiological evidence relative to food safety.
29.3.1 Application
The application of HACCP principles consists of the following tasks as the Logic Sequence for Application of HACCP
29.3.2 Principles
The HACCP system consists of the following seven principles:
Principle 1 : Conduct a hazard analysis.
Principle 2 : Determine the Critical Control Points (CCPs).
Principle 3 : Establish critical limit(s).
Principle 4 : Establish a system to monitor control of the CCP.
Principle 5 : Establish the corrective action to be taken when monitoring indicates that a particular CCP is not under control.
Principle 6 : Establish procedures for verification to confirm that the HACCP system is working effectively.
Principle 7 : Establish documentation concerning all procedures and records appropriate to these principles and their application.
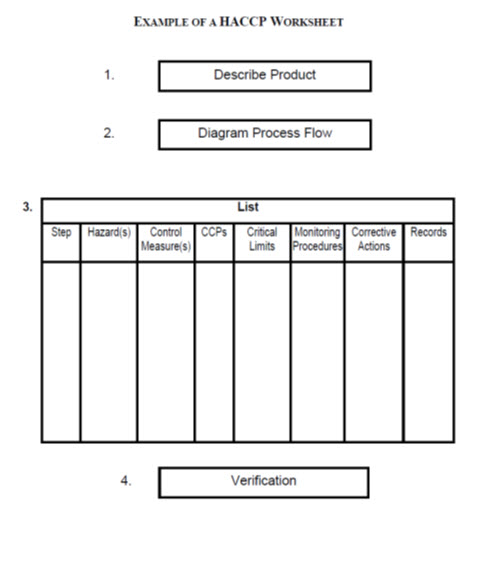
29.3.3 Logic Sequence for Application of HACCP
Assemble the HACCP Team
Describe the Product
Identify Intended Use
Construct Flow Diagram
On‑site Confirmation of Flow Diagram
List All Potential Hazards Conduct a Hazard Analysis Determine Control Measures
Determine CCPs
Establish Critical Limit for Each CCP
Establish a Monitoring System for Each CCP
Establish Corrective Action for Deviations that May Occur
Establish Verification Procedures
Establish Documentation and Record Keeping
29.3.3.1 Assemble HACCP team
The food operation should assure that the appropriate product specific knowledge and expertise is available for the development of an effective HACCP plan. This is accomplished by assembling a multidisciplinary team consisting production, QA, engineering, marketing, purchase, HR personnel. The scope should describe which segment of the food chain is involved and the general classes of hazards to be addressed.
29.3.3.2. Describe product
A full description of the product should be drawn up, including relevant safety information such as: composition, physical/chemical structure (including aw, pH, etc.), microcidal / static treatments (e.g. heat-treatment, freezing, brining, smoking, etc.), packaging, durability and storage conditions and method of distribution.
29.3.3.3. Identify intended use
The intended use should be based on the expected uses of the product by the end user or consumer. In specific cases, vulnerable groups of the population, e.g. institutional feeding may have to be considered.
29.3.3.4. Construct flow diagram
The flow diagram should be constructed by the HACCP team. The flow diagram should cover all steps in the operation. When applying HACCP to a given operation, consideration should be given to steps preceding and following the specified operation.
29.3.3.5. On‑site confirmation of flow diagram
The HACCP team should confirm the processing operation against the flow diagram during all stages and hours of operation and amend the flow diagram where appropriate.
29.3.3.6. List all potential hazards associated with each step, conduct a hazard analysis and consider any measures to control identified hazards
The HACCP team should list all of the hazards that may be reasonably expected to occur at each step from primary production, processing, manufacture, and distribution until the point of consumption.
The HACCP team should conduct a hazard analysis to identify for the HACCP plan which hazards are of such a nature that their elimination or reduction to acceptable levels is essential to the production of a safe food. The team must then consider what control measures, if any, exist which can be applied for each hazard.
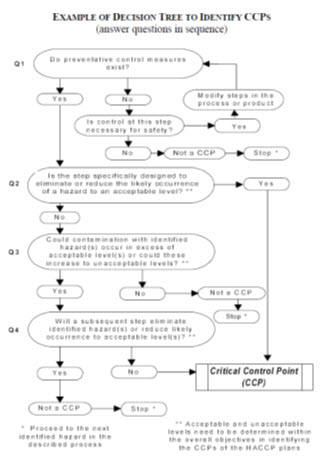
29.3.3.7. Determine Critical Control Points
There may be more than one CCP at which control is applied to address the same hazard. The determination of a CCP in the HACCP system can be facilitated by the application of a decision tree which indicates a logic reasoning approach. Application of a decision tree should be flexible, given whether the operation is for production, slaughter, processing, storage, distribution or other. It should be used for guidance when determining CCPs.
29.3.3.8. Establish Critical Limits for each CCP
Critical limits must be specified and validated if possible for each critical control point. In some cases more than one critical limit will be elaborated at a particular step. Criteria often used include measurements of temperature, time, moisture level, pH, Aw, available chlorine and sensory parameters such as visual appearance and texture.
29.3.3.9. Establish a Monitoring System for each CCP
Monitoring is the scheduled measurement of a CCP relative to its critical limits. The monitoring procedures must be able to detect loss of control at the CCP. Data derived from monitoring must be evaluated by a designated person with knowledge and authority to carry out corrective actions when indicated. Physical and chemical measurements are often preferred to microbiological testing because they may be done rapidly and can often indicate the microbiological control of the product. All records and documents associated with monitoring CCPs must be signed by the person doing the monitoring and by a responsible reviewing official of the company.
29.3.3.10. Establish a Corrective Actions
Specific corrective actions must be developed for each CCP in the HACCP system in order to deal with deviations when they occur. The actions must ensure that the CCP has been brought under control. Actions taken must also include proper disposition of the affected product. Deviation and product disposition procedures must be documented in the HACCP record keeping.
29.3.3.11. Establish a Verification Procedures
Verification and auditing methods, procedures and tests, including random sampling and analysis, can be used to determine if the HACCP system is working correctly. Examples of verification activities include:
Review of the HACCP system and its records
Review of deviations and product dispositions
Confirmation that CCPs are kept under control
29.3.3.12. Establish a Documentation and Record Keeping
Efficient and accurate record keeping is essential to the application of a HACCP system. HACCP procedures should be documented. Documentation and record keeping should be
appropriate to the nature and size of the operation.The HACCP system is to focus control at Critical Control Points ( CCP). Redesign of the operation should be considered if a hazard which must be controlled is identified but no CCPs are found. HACCP should be applied to each specific operation separately. The HACCP application should be reviewed and necessary changes should be made when any modification is made in the product, process, or any step.
29.4 Terminology
Control (verb):
To take all necessary actions to ensure and maintain compliance with criteria established in the HACCP plan.
Control Measure:
Any action and activity that can be used to prevent or eliminate a food safety hazard or reduce it to an acceptable level.
Corrective Action:
Any action to be taken when the results of monitoring at the CCP indicate a loss of control.
Critical Control Point (CCP):
A step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level.
Critical Limit:
A criterion which separates acceptability from unacceptability.
Deviation:
Failure to meet a critical limit.
HACCP:
A system which identifies, evaluates, and controls hazards which are significant for food safety.
HACCP Plan:
A document prepared in accordance with the principles of HACCP to ensure control of hazards which are significant for food safety in the segment of the food chain under consideration.
Hazard:
A biological, chemical or physical agent in, or condition of, food with the potential to cause an adverse health effect.
Hazard Analysis:
The process of collecting and evaluating information on hazards and conditions leading to their presence to decide which are significant for food safety and therefore should be addressed in the HACCP plan.
Monitor:
The act of conducting a planned sequence of observations or measurements of control parameters to assess whether a CCP is under control.
Validation:
Obtaining evidence that the elements of the HACCP plan are effective.
Verification:
The application of methods, procedures, tests and other evaluations, in addition to monitoring to determine compliance with the HACCP plan.