Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Lesson 5. Colloids - I
5.1 INTRODCTION
Thomas Graham (1861) while doing his classical researches on liquid diffusion observed two classes of substances.
Solution of some substances could readily pass through animal and vegetable membranes. To this class belonged almost all inorganic acids, bases and salts and many organic compounds like urea, sugar etc. Graham called them crystalloid as most of them were crystalline.
The other class of substances are diffused very slowly in solution and the solution could not pass through the membrane, they were called colloids (from the Greek word Kolla meaning glue, which is a member of this class). Other members are starch, gelatin, albumin, proteins and other amorphous substances.
Graham’s view has undergone changes since it has been seen that every substance can exhibit colloidal properties under suitable conditions. Thus we now define colloidal state as: a two phase heterogeneous system in which one phase is dispersed in a fine state of subdivision having diameter of 10-4 to 10-7 cm in another medium termed the continuous or dispersion medium. In a colloidal solution there exists a discontinuous phase called the dispersed phase and the continuous dispersion medium, and also a stabilizing agent which does not allow the dispersed phase to coalesce and settle.
5.2 CLASSIFICATION OF COLLOIDS
5.2.1 Classification based on dispersed phase and dispersion medium which may be either solid or liquid or gas and so the following types (Table 5.1) of colloids are formed
Table 5.1 Types of colloidal systems
| Dispersion medium | Dispersed phase | Colloidal system | Examples |
| Gas | Liquid Solid | Liquid aerosol Solid aerosol | Mist, Fog, Cloud, Insecticide spray, Smoke, Fume |
| Liquid | Gas Liquid Solid | Foam Emulsion Sol | Foam, Froth, Whipped cream, Lemonade Milk, Cream, Mayonnaise, Glue, Paints, Starch paste, Milk of Magnesia, Gold sol, As2O3, S, FeO3 or AgCl sol in water |
| Solid | Gas Liquid Solid | Solid foam Gel Solid sol | Pumice stone, bread dough, Lava, Styrene foam, Rubber. Curd, Jelly, Cheese, Butter, Shoe polish, Pearl (CaCO3 with water). Gems, Alloys, Minerals |
5.2.2 Classification based on affinity for solvent When there is a strong affinity between the dispersed phase and the dispersion medium the colloid is known as lyophilic colloid, while if there is no apparent affinity it is called lyophobic colloid. The main differences between these two classes of colloids are summarised below:
Lyophillic sol are stable. The particles may or may not migrate in an electrical field, and viscosity is much higher than that of the medium. They are reversible sols and are self-stabilized due to the strong attraction between two phases. They are self-stabilized due to the strong attraction between two phases. Surface tension is much lower than that of the medium. They are not easily precipitated by the addition of electrolytes. Starch gelatin, gum, albumin are lyophilic colloids which are not visible under ultramicroscope. They are prepared simply by direct dissolving.
Lyophobic sols are unstable compared to lyophillic sols. The particles are charged and hence move towards electrode in an electrical field. The viscosity is same as that of the medium. They are irreversible sols. They are much less stable, stabilizer is required for stabilization. Surface tension is same as that of the medium and is coagulated easily by adding electrolyte. Colloidal metals, metallic sulfides are lyophobic colloids, Visible under ultramicroscope and prepared by indirect methods
5.2.3. Classification based on nature of dispersed phase
i. Multimolecular colloids: When the dispersed phase consists of aggregates of atoms or molecules and the main attractive force between them is the van der Waals force of attraction. They are lyophobic in nature.
Examples: Gold sol and Sulfur sol.
ii. Macromolecular colloids: A large number of small molecules combine to form a giant molecule held by covalent bonds having a colloidal dimension are called macromolecular colloids. They are lyophilic and have gold numbers.
Examples: Proteins, Polysaccharides, Gums, Synthetic rubber, Nylon etc.
iii. Association colloids: On increasing concentration, the solute molecules or ions come closer to form aggregate spontaneously of colloidal dimensions, called micelles are associated colloids. Example: Soaps and synthetic detergents.
Sodium oleate forms micelles and contains a lyophobic group (C17H33+) and a lyophilic group (-COONa), the hydrocarbon portion inside the micelle, while the ionised groups are at the surface. They move freely in an electric field and the solution has high conductivity. The minimum concentration at which the micellization begins is called the critical micellization concentration (cmc). These colloids play very important role in solubilizing solvent repellant substances, such as water insoluble dyes are solubilized in soap solutions.
5.3 PREPARATION OF COLLOIDAL SOLUTIONS
The main methods of preparation of colloidal solutions are
5.3.1. Condensation method
5.3.2. Dispersion method
5.3.1 . Condensation Method: In this method, a large number of particles of molecular size are allowed to coalesce or condense to form bigger particle of colloidal dimension. The various processes used are:
i. By exchange of solvent: Sulfur or phosphorus is not directly soluble in water. If an alcohol solution of S or P is poured into water, colloidal solution of S or P is formed.
ii. By changing physical state: Hg or S sols are produced by allowing their vapours to pass through cold water. Ammonium citrate is added to stabilize the solution.
iii. By controlled condensation: Prussian blue sol is obtained by precipitating it in presence of a protective colloidal system like starch.
iv. Chemical methods: (a) by double decomposition, colloidal solution of arsenious sulfide is made by passing H2S gas, through a suspension of As2O3in water, until saturation
As2O3 + 3H2S ⎯⎯→ As2S3 + 3H2O.
Sols of insoluble inorganic salts are prepared in this way
b. By reduction: Colloidal solutions of metals such as Ag, Au, Pt in water can be prepared by reducing aqueous solution of their salts by reductants like formaldehyde, carbon monoxide, hydrazine etc
Thus gold sol is obtained by treating a solution of gold chloride with formaldehyde
(0.3 %).
2AuCl3+ 3HCHO + 3H2O à 2 Au(sol) + 3HCOOH + 6HCl
The liberated acid is neutralized by K2CO3to stabilize the sol.
c. By hydrolysis: Colloidal solutions of many oxides and hydroxides of iron, aluminium can be prepared by this method. On adding a freshly prepared saturated solution of FeCl3 dropwise on boiling water, hydrolysis of FeCl3 takes place with the for
mation of deep red colloidal sol of ferric hydroxide.
FeCl3 + 3H2O à Fe(OH) 3 (sol) + 3HCl
d. By oxidation: Sulfur sol is prepared by passing H2S through a solution of SO2 in wate until the smell of SO2 is removed. The solution on boiling with satrated NaCl solution precipitates out S, on filtration, which is washed free of Cl- and finally dissolves on further addition of water.
5.3.2. Dispersion method: In this method, the bigger or large particles are broken down to colloidal dimension and dispersing them in a medium to form colloidal solution. The methods used are:
i. By mechanical dispersion: Many substances of large size are ground and dispersed in a medium to get a coarse suspension which is then passed through a colloid mill, which is essentially two metal discs rotating in opposite directions at high speed with a very narrow clearance
The space in between the metal discs breaks down the particles to colloidal size. Black ink, paints, varnishes, ointments dyestuffs are prepared by this method
ii. Bredig’s arc method or electro dispersion: (Figure 5.1) Colloidal solution of metals like Au, Ag, Cu, Pb, Pt etc. are obtained by this method. An electric current is passed through water containing traces of alkali, when an electric arc is produced between the tips of two closely placed electrodes of these metals. The heat of the spark converts the ends of the metal rods to form vapours of metal which immediately condense to give metal colloids. The trace of alkali stabilizes the colloidal solution and finally the solution is cooled by cooling mixture.
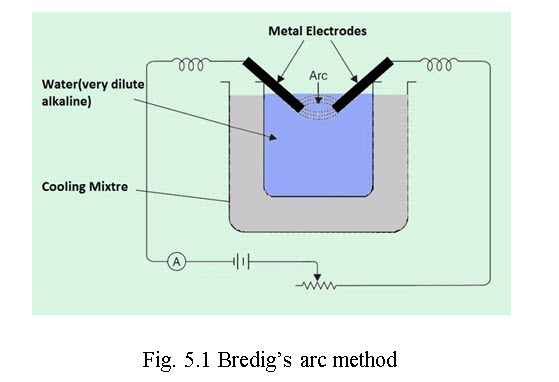
Fig. 5.1 Bredig’s arc method
iii. Mechanical dispersion method: (Fig. 5.2) this technique consists of finely pulverising the substances to be dispersed in the medium, which constitutes the dispersion phase. This coarse suspension is then passed through colloid-mill which mechanically grinds the coarse particles. The colloid-mill consists of two discs made of metal, which rotates in opposite direction at a very high speed. The clearance between the discs is very small that apparently determines the size of the colloidal particle. The coarse particles which come in between the two rotating discs get teared to smaller colloidal particles, which are finally stabilized by addition of a suitable protective colloid.
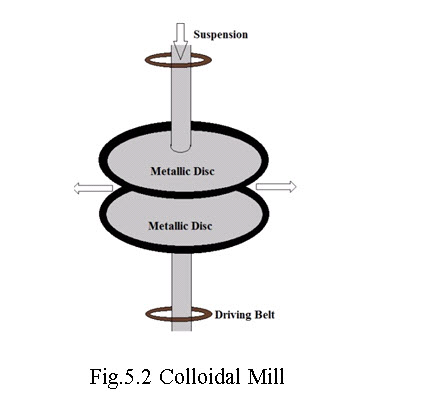
Fig.5.2 Colloidal Mill
iv. Peptization: A freshly prepared precipitate such as AgCl, Fe(OH)3 , Al(OH) 3 etc can be converted into colloidal state by the addition of small amount of a foreign electrolyte solution. The foreign electrolyte is known as the peptizing agent and the process is called peptization.
a. Thus freshly precipitated Fe(OH) 3, if shaken with a dilute solution of FeCl3, would
give rise to ferric oxide sol.
Fe(OH) 3 (s) + Fe3+ → Fe(OH) 3 . Fe3+
Precipitate Peptizing agent (Sol/Colloid)
b. Freshly precipitated AgCl can be peptized by shaking with a dilute aq sol. of AgNO3
AgCl(s) + Ag+ → AgCl . Ag+
Precipitat Peptizing agent (Sol/Colloid)
c. Al(OH)3 + H+ →Al(OH)3 . H+
Precipitate Peptizing agent (Colloid)
Though there is no rule, but it is common that the peptizing agent which are most effective contains a common ion with the precipitate forming colloid.
Dispersion is also sometimes carried out by the use of an atomiser e.g., in the dispersion of a liquid in a gas.
5.4 PURIFICATION OF COLLOIDAL SOLUTIONS
The colloidal solutions prepared by the above methods contain several impurities of electrolytes and other soluble substances. So, they become unstable. Hence, it is essential to purify these colloidal sols. The following methods are commonly used for purification.
i. Dialysis. The semi-permeable membranes like cellophane parchment paper, animal membrane have pore sizes which allow the free passage of only the dissolved molecules or ions of a true solution but retains the bigger colloidal particles. Thus, the process of separation of crystalloids from colloids by diffusion through the animal or vegetable membrane is called Dialysis. The sol to be purified is taken in a parchment or cellophane bag, which is partly immersed in water or the dispersion medium. The crystalloids from the sol move out through the cellophane while some amount of water or solvent from outside may enter the bag. The solvent is renewed from time to time. The process is allowed to continue until most of the ions are eventually removed and the retained colloid gets stabilized. In certain sols such as Al2O3 hydrosol, the disperse phase is too small to be retained by cellophane and hence the system needs controlled heating whereby the particles grow bigger in size.
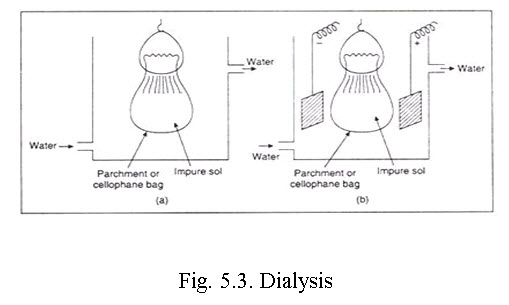
To accelerate this slow process of purification and also to achieve a higher degree of purity the dialysis is carried out in a direct current electric field, and the process is called electrodialysis. Electrodialysis is of no use in separating non-electrolytic impurities.
ii. Ultra-filtration. Colloidal particles can pass through ordinary filter papers. But when ordinary filter paper is impregnated with a solution of gelatin or collodion and hardened by formaldehyde the pores become smaller, so that colloidal particles cannot pass through it. Such a filter paper is called ultrafilter paper. The sol when poured over the ultrafilter, electrolytes can pass through, but the colloidal particles are retained and form a slime over it. The slime when brought in contact with water, disperses freely and forms colloidal solution. This process of purifying colloids from the crystalloids using ultrafilters is called ultrafiltration.
By using impregnating solutions of different concentration graded ultrafilters can be prepared which is capable of separating impurities of different sizes.
Reference