Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Lesson 6. Colloids - II
6.1 PROPERTIES OF COLLOIDAL SOLUTIONS
Colloidal solutions possess some characteristic properties. Important ones are described below:
6.2.1 PHYSICAL PROPERTIES
a. Colloidal systems are heterogeneous, intermediate between true solution and suspension and contain two phases, dispersed phase and dispersion medium.
b. Colour. Colour and appearance depend on the size, shape, refractive index and some other properties of the dispersed phase. Gold sol of very fine particles is red and of coarser particles is blue in colour; sulfur sols may be colorless to faint and deep yellow in reflected light and reddish in transmitted light.
c.Shape. Although the most common shape of the particles are spherical; but highly asymmetrical shape is also found. Vanadium pentoxide particles are rod-shaped, RBCs (red blood corpuscles) normally are double concave plates and give red color, but in sickle-cell anaemia they become new-moon shaped. Colloidal particles are readily filterable through ordinary filter paper.
d. Adsorption. Generally colloidal particles are either positively or negatively charged, so they attach to a variety of molecules on their surfaces. This property of colloid has been utilized in dyeing fabrics where the fabrics are first treated with colloidal suspension which adsorbs dye.
6.2.2 COLLIGATIVE PROPERTIES.
High molecular weight is the characteristic of colloidal particles and hence in a solution, the number of moles present is very low. Colligative properties like osmotic pressure, depression of freezing point, lowering of vapour pressure depend on the molarity of the solution and hence these values are very low for colloidal solution.
6.2.3 MECHANICAL PROPERTIES
a. Brownian movement. Robert Brown, an English botanist, in 1927, observed under a microscope that pollen grains dispersed in water executed a ceaseless random motion. This random zigzag motion is also executed by all colloidal particles, including colloidal particles suspended in a gaseous medium, independent of their nature, smaller particles executing a more rapid and brisk motion than the larger ones. This perpetual zigzag motion is called Brownian motion. This motion is described by Perrin as .they go and come, stop, start again, mount, descent, remount again, without in the least tending towards immobility.
The true cause of Brownian movement was suggested by Wiener in 1863 as molecular collision of colloidal particles with liquid molecules. The colloidal particles are constantly hit from all sides of the surrounding molecules. The colloidal particles after impact with solvent molecule move in a straight line until its speed and direction are not altered by another impact. The intensity of Brownian motion is less with increase in size of the colloidal particles and also with the increase in viscosity of medium.
Brownian motion also increases with increase in temperature. Large particles do not show Brownian movement.
b. Diffusion. In a colloidal solution, colloidal particles move from a region of higher concentration to a region of lower concentration until uniformity is attained. But the diffusion process is slower than diffusion in true solution due to higher mass and lower speed of colloidal particles. Diffusion is used to separate colloidal particles of different size.
c. Sedimentation. Colloidal particles constantly exhibit Brownian motion and remain suspended in solution, thereby stabilizing the colloidal solution. Larger colloidal particles settle slowly by the effect of gravity and is called sedimentation. Svedberg increased the rate of sedimentation with centrifugal sedimentation by employing a high-speed centifuge called ultracentrifuge. In this machine sedimentation is effected under enormous centrifugal force of rotation.
6.2.4 OPTICAL PROPERTIES OR TYNDALL EFFECT
When a beam of light is passed through a colloidal solution, the path of light becomes illuminated due to the scattering of light by the colloidal particles. This phenomenon is called Tyndall effect, which does not happen for true solution. These colloidal particles can be viewed through a microscope placed at right angles to the path of light and is called the ultramicroscope. The phenomenon of Tyndall effect can be explained by the scattering of light by the colloidal particles. The particles absorb the incident light energy, becomes self-luminous and scatter this absorbed light. The intensity of the scattered light depends on the difference between the refractive indices of particle and medium.
Tyndall effect was first studied by Zeigmondy in 1903 and this effect can be applied for viewing the colloidal particles quantitatively.
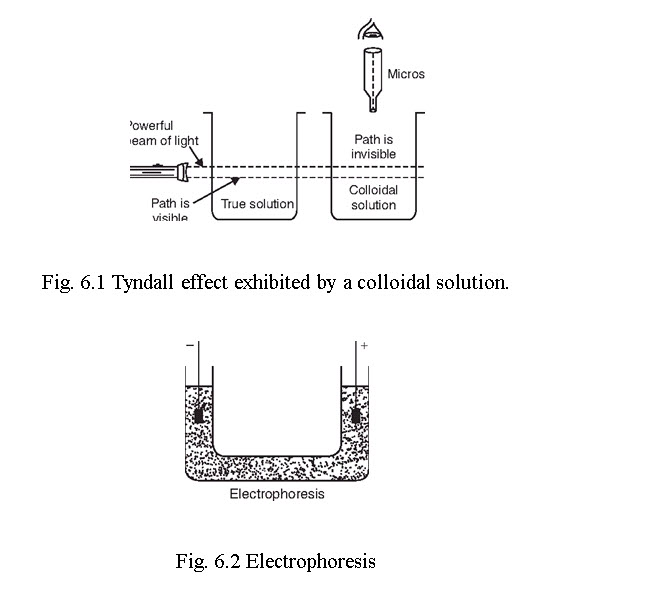
6.2.5 ELECTRICAL PROPERTIES
-
Electrophoresis. Lyophilic and lyophobic colloid particles are either positively or negatively charged. In a U-tube, containing a colloidal solution, when a potential gradient is applied the colloidal particles move towards oppositely charged electrode. After reaching the electrode, they lose their charge and coagulate there. The movement of the colloidal particles in an electric field is called electrophoresis. When the negatively charged particles like CdS, As2S3 metallic sols, organic sols move towards cathode, the phenomenon is called cataphoresis. Positively charged particles are Fe(OH)3, Al(OH)3, basic dyes etc. The speed of the colloidal particle under the potential gradient of 1 volt/cm is called electrophoretic mobility.
-
Electro-osmosis. When electric current is passed through a colloidal solution such that the dispersed phase is prevented from movement, the dispersion medium begins to move in the electric field. This phenomenon is called electro-osmosis.
-
Isoelectric Point. The colloidal sols of protein are either positively or negatively charged in acidic or alkaline solution and hence when electrolysed in a medium, pH of the medium will dictate the migration of the colloidal particle to any electrode. Hence the pH of the dispersion medium at which the dispersed particles are neutral and hence they do not migrate when subjected to an electric field is called isoelectric point. The isoelectric point of protein depends on pH and ionic strength in the medium. Proteins are least soluble in isoelectric point and hence coagulate at isoelectric point.
6.3 COAGULATION OF COLLOIDS
Colloid particles are electrically charged, so they repel each other and become stable. When the charge is neutralised, the particles approach each other to form aggregates and settle down. The precipitation of colloidal solution is called coagulation or flocculation. This can be achieved by
Adding oppositely charged: Two oppositely charged colloidal solutions when mixed, mutual coagulation of the two sols takes place.
Electrophoresis: In electrophoresis the electrically charged colloidal particles migrate towards the oppositely charged electrode and finally coagulate.
Adding electrolytes: By addition of oppositely charged ion i.e., by adding electrolytes the charges of the colloidal particles are neutralised and precipitated. Coagulating power of an ion increases with the increasing valency of the coagulating ion. The higher the valency of the ion, greater is the coagulating power this is known as Schulze-Hardy law. Thus, the coagulating power increases in the series
Na+ < Ba+2 < Al3+ or Cl- < SO42− < PO43- .
Generally, the coagulating power of trivalent ion is 500 - 1000 times greater than bivalent ion which is 100-500 times greater than monovalent ion.
6.4 PROTECTION OF COLLOID
The stability of colloids can be achieved by any of the following:
Electrical charge. All the colloidal particles of a colloidal solution bear similar charges and hence they repel each other. This mutual repulsion prevents them from coalescing and coagulating.
Solvation. Colloidal particles are surrounded by a layer of solvent molecules which resists the dispersed phases from forming large aggregates and coagulate. Thus, the solvent layer forms a protective envelope. These sols are very stable.
Protective agents. Protective agents are added to some colloidal systems, which get adsorbed on the surface of the colloidal particles and by coating them prevents coagulation.
Protective colloids. Lyophilic colloids are much more stable than the lyophobic colloids.since the former are extensively solvated. When a lyophilic colloid is added to a lyophobic colloid, the latter becomes more stable even to the action of electrolytes. The process by which lyophilic colloid protects the lyophobic sol is called protection and the lyophilic colloid is called the protective colloid. Thus, if a little albumin (lyophilic colloid) is added to a gold sol (lyophobic sol), the former acts as protective colloid and prevents coagulation of the latter even against addition of high concentration of an electrolyte, like NaCl. This protection is due to the formation of a protective thin layer surrounding the particles of the lyophobic colloid, through which the oppositely charged ion of the electrolyte cannot penetrate to neutralize the charge for coagulation. Zsigmondy introduced the term gold number to measure the protective power of lyophilic colloids. It is defined as the minimum weight in mg of a protective colloid, which when added to one ml of standard gold sol (containing 0.0053 to 0.0058 % gold) containing also 1 ml of 10 % NaCl solution to just prevent its coagulation. This is indicated by the change of colour from red to blue. The smaller the gold number the greater is the protective power of lyophilic colloid.
Table 6.1 Gold numbers
|
Protective colloid |
Gold number |
|
Gelatin Casein Albumin Gum Starch Sodium oleate Haemoglobin |
0.005 to 0.015 0.01 to 0.02 0.1 to 0.2 0.1 to 0.2 10 to 15 0.40 0.003 to 0.07 |
6.5 EMULSION AND GEL
Emulsions are colloidal systems in which both dispersed phase and dispersion medium are liquids (immiscible). The familiar examples are milk, which consists of liquid fat dispersed in water (oil in water type); butter, which consists of water dispersed in liquid fat (water in oil type).
Emulsions are prepared merely by shaking one liquid with the other, and hence they are not stable and easily separate into two immiscible layers. Emulsions are stabilized by adding a third substance (emulsifier) as stabilizer. This substance forms a thin layer around the dispersed phase thereby preventing it from coalescing and coagulating. The emulsifier also decreases the surface tension of the two liquids.
Albumin, casein, gum, agar-agar, soaps and detergents are the common emulsifiers. Emulsifiers are selected according to the type of emulsion, e.g., soaps of alkali metals, gelatin etc. are more soluble in aqueous phase, hence they are used as stabilizers for oil-in-water-type of emulsion whereas heavy metal soaps of palmitic and oleic acid are soluble in fat and hence used in water-in-oil-type emulsion.
Emulsions have properties similar to those of lyophobic colloids like Tyndall effect, Brownian movement etc.
Gel: Many lyophilic sol and few lyophobic sol when coagulated under certain conditions form semi-solid mass. Such a product is called gel and the process is called gelation, e.g., jam, jelly, cheese, curd etc. This is formed by entrapment of the dispersion medium inside the loose network of the dispersed particle. Gels have high viscosity and swell if placed in the dispersion medium and some shrink in volume.
Gel formation is utilized for preparation of shoe polish, cheap soaps, curds, jellies, solid fuel of alcohol with calcium oxalate.
Reversible colloid. When a colloid can be reversibly converted from a sol to gel and vice versa, it is called a reversible colloid, e.g., solution of gelatin can be reconverted into sol by heating. Other colloids which cannot be reversibly transformed are called irreversible colloid, e.g., normal Au colloid.
Zeta potential. In lyophobic colloids, particles are of similar charge, which do not allow the particles to come closer and coalesce and hence bring stability to the system.
The charge is embedded in the colloidal particle and an equivalent amount of opposite charge remains outside and these two layers form a double layer. A part of this opposite charge is fixed on the particle and another part is mobile and distributed throughout the medium.
Hence, there is a potential difference between the particle and the medium. This potential is called the zeta potential which is responsible for the stability of the colloid.
6.6 APPLICATION OF COLLOIDS
Purification of water. Impure water is treated with alum. Al3+ ions of alum precipitate negatively charged clay particles, bacteria, colloidal particles and the impurities.
Purification of polluted air. Suspended particles, smoke etc., polluted air. Smoke is negatively charged colloidal suspension consisting of carbon particles. Air is passed through Cottrell precipitator where the charge is neutralised and carbon settles down while the hot air passes out through chimney.
Removal of dirt from sewage. Sewage contains charged dirt particles, which is passed through tanks fitted with oppositely charged electrodes. By neutralizing the charge, dirt is coagulated.
Leather tanning. The raw material skin is positively charged colloidal system, which is treated with negatively charged colloidal solution of tannin, whereby mutual coagulation of oppositely charged colloids takes place.
Laundry. Soaps in water form colloidal solution, which adsorb greasy materials, dirt by emulsion formation and remove dirt from clothes.
Medicine. Colloidal system of medicines are better assimilated. Blood coagulation is effected with alum or FeCl3.
Cosmetics. Different types of colloidal systems and emulsion are used in cosmetics.
Analysis. Noble metals are detected by formation of their colloidal solutions and observing their colours.
Artificial rains are made by throwing charged sand on clouds.
Delta formation. Negatively charged sand particles of the river water Sand Na+, K+, Ca2+ present in the sea water are responsible for the deposition of colloidal particles where river meets sea.