Site pages
Current course
Participants
General
Module 1. Dairy Development in India
Module 2. Engineering, thermal and chemical proper...
Module 3. Unit operation of various dairy and food...
Module 4. Working principles of equipment for rece...
Module 5. Dairy plant design and layout, compositi...
Module 6. Deterioration in products and their cont...
Module 7. Physical, chemical and biological method...
Module 8. Changes undergone by the food components...
Module 9. Plant utilities requirement.
References
Lesson 5. Chemical properties of milk and milk products-II
5. INTRODUCTION
The physico-chemical properties of milk and milk products affect most of the unit operations used during their processing. These operations include fluid flow, heat transfer processes, mixing and churning, emulsification and homogenisation. Some of the rheological properties are also used for assessing and monitoring the quality of products such as cream. dahi, butter and cheese.
Raw milk is extremely variable in its composition and most dairy products can be produced in a variety of ways from this milk. There are two approaches to obtain, data for physical properties. The first is to use data available in the literature; the second is to determine the values experimentally.
5.1 Refractive Index
The refractive index of milk is sometimes used to indicate adulteration especially watering. The refraction of light by a solution depends upon the individual molecular substance present and upon their concentrations. The total refraction is sum of the individual refractions of the constituents present in the solution. The refractive index of the milk then is the refractive index of the solvent plus the indices of the solutes. The freezing point determination is more reliable than the refractive index for detecting added water. The average refractive index of cow milk has been found to be 1.3461 and that of buffalo milk 1.3477 at 40 ̊C.
5.2 Acidity and pH
The natural or apparent acidity of fresh milk is caused by the presence of casein, acid-phosphates, citrates etc. in milk. Thus higher the solids-not-fat the higher the natural acidity. The natural acidity of cow milk varies from 0.13 - 0.14 percent and that of buffalo milk from 0.14 – 0.15 percent. Developed or real acidity is due to lactic acid formed as a result of bacterial action on milk lactose. The titrable acidity is usually expressed as percent of lactic acid and for stored milk is equal to the sum of natural acidity and developed acidity.
As milk is slightly acidic, its pH is lower than 7 which is the value for neutrality. The pH of fresh cow milk varies from 6.6 to 6.7 and that of buffalo milk from 6.7 to 6.8 Skimming and dilution with water raise the pH of milk while sterilization usually lowers it.
Soxhlet-Henkel value (potential acidity or titrable acidity). The acid reaction as it occurs in milk and dairy products is often expressed in terms of the Soxhlet-Henkel value (SH, SHV),
The SH value indicates how many cm3 of a caustic solution with a concentration c (NaOH) of 25 mol per cm3 (N/4) are required to neutralize 100 cm3. As an indicator, about 1 cm3 of a 2% alcoholic phenolphthalein solution is added. Absolutely fresh milk from healthy cows has an SH value of 6.4-7.0. Part of this is due to acidic salts, e.g., NaHC03, and the free organic acids (mainly citric acid); the balance is due to casein, which reacts to NaOH like an acid. The increase of the SH value after milk coagulation is due to the formation of lactic acid by the corresponding bacteria, especially lactic acid bacteria.
SH values in raw milk < 5.0 indicate mastitis, defects in feeding or negative microbial influences, resulting in reduced lab coagulation or taste defects in milk. SH values of 8.0- 9.0 result in a noticeable sour taste, at SH values> 10 protein coagulation during heating of the milk occurs.
SH values of 10-12 will not necessarily lead to heat precipitation, because due to breeding activities a higher value can be obtained. Then the acidity has to be determined by the pH value, as the SH analysis cannot determine the dissociated part of the acid precisely.
As the dissociated part of the acid cannot be determined precisely by SH analysis (and nondissociated acidic parts can also be neutralized too), we call this the probable potential acidity. This determination becomes more imprecise the lower the percentage of titrable serum becomes (e.g., cream with a high fat content).
Other acidity determinations by titration result in Thorner degrees and the Domic grade. Sometimes acidity is given as g lactic acid per 100 cm3 milk or % lactic acid
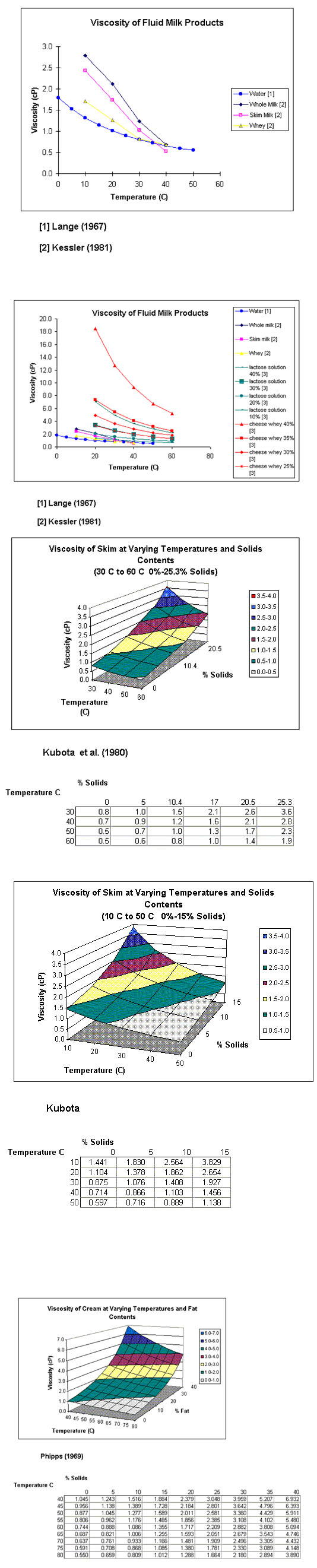
5.3 Viscosity
The dynamic viscosity μ is a parameter related to the inner friction of a liquid or fluid. It is reduced when temperature is increased. Due to the friction of the fat (emulsified in milk) and the dispersed protein, the viscosity of milk is twice as high as that of water. It increases with the protein coagulation and increasing fat content. The dimension of the dynamic viscosity is Ns/m or Pa-s; an old term is the centipoises cP (10-3 Pa-s). The value for milk at 5°C is a function of the fat content and ranges from 2.96 X 10-3 Pa- s (skimmed milk) and 3.25 X 10-3 Pa-s (whole milk); at 20°C we observe a range of 1.79 X 10-3 Pa-s and 2.13 X 10-3 Pa-s.
5.4 Surface Tension
Surface tension σ [N/m] is often given in dyne/cm (1 dyne/cm = 10 N/m); it is the work W required to recreate a new liquid surface A of 1cm. In other terms, the surface tension corresponds to a force F which is oriented parallel to the surface, in relation to 1cm of length l, against which work is to be done when the surface is increased.
The surface tension, which causes the contraction of a liquid surface, acts as an inter- face tension between phase layers (liquid/air, liquid/liquid, liquid/solid). Each phase has its own surface tension, which depends upon the characteristics of the ingredient. In cleaning processes e.g., the surface tension of the area to be cleaned is reduced, which results in complete wetting of the surface despite the higher surface tension of the water. The surface tension of water is 72.8 dyne/cm at 20°C. Milk and dairy products have a lower value due to fat and protein. Surface tensions for milk and dairy products (all data in dyne/cm) are:
Raw milk 49 – 51 Standardized milk 51
Skimmed milk 52 – 53 Cream 42 – 45
Buttermilk 39 – 40 Lab whey 51 - 52