Site pages
Current course
Participants
General
MODULE 1. Magnetism
MODULE 2. Particle Physics
MODULE 3. Modern Physics
MODULE 4. Semicoductor Physics
MODULE 5. Superconductivty
MODULE 6. Optics
LESSON 7. Heisenberg Uncertainty Principle
Heisenberg Uncertainty Principle
Heisenberg explained a principle which is aim toward importance of dual nature of the matter.
According to classical mechanics
- Suppose a particle is moving, so at any instant has a Fixed Position in space and definite momentum can be measured if the initial value are known.
But according to wave mechanics,
A particle is considered as a wave packets.
Now suppose, wave packet (concept of \[{\rm{\lambda }}\]) is small, the position of particle may be fixed but velocity or momentum of particle become indefinite.
In other way, wave packet is large, the velocity or momentum may be fixing and position of particle is indefinite.
Thus, certain (definite) position of the particle has uncertainty in momentum or velocity and certain momentum and velocity has uncertain in position of particle.
From these probabilities it is impossible to know
Where within the wave packet the particle is?
What is its exact momentum or velocity?
According to Heisenberg uncertainty principle
It is impossible to specify precisely and simultaneously the value of both of physical variables that describe the behavior of an atomic system.
Qualitative principle states
The order of magnitude of the product of the uncertainties in the knowledge of two variables must be at least Plank’s Constant
Let pair of two variables (physical variables) as position of particle and momentum of particle.
Δx Δp = h
Δx - Uncertainty in determining the position of particle.
Δp - Uncertainty in determining the momentum of particle
ΔE Δt = h
ΔE - Uncertainty in determining the energy
Δt - Uncertainty in determining in determining the time
Δj Δ\[\theta\] = h
Δj - Uncertainty in determining angular momentum
Δ\[\theta\] - Uncertainty in determining angle
Experimental Justification of uncertainty Principle
(1) Determining of the position of a particle by microscope
For the measured of position of a particle (electron) in the range of microscope the resolving power of the microscope can be measure the smallest distance between the two points,
Δ\[x={\lambda\over{2\sin \theta }}\]……..(1)
Δx - Uncertainty in determining the position of particle
\[\lambda\] - Wavelength of light used
\[\theta\] - Semi vertical angle of the cone of light
To observed the particle (position of electron), it is necessary a photon strikes the electron (particle) and scattered inside the microscope.
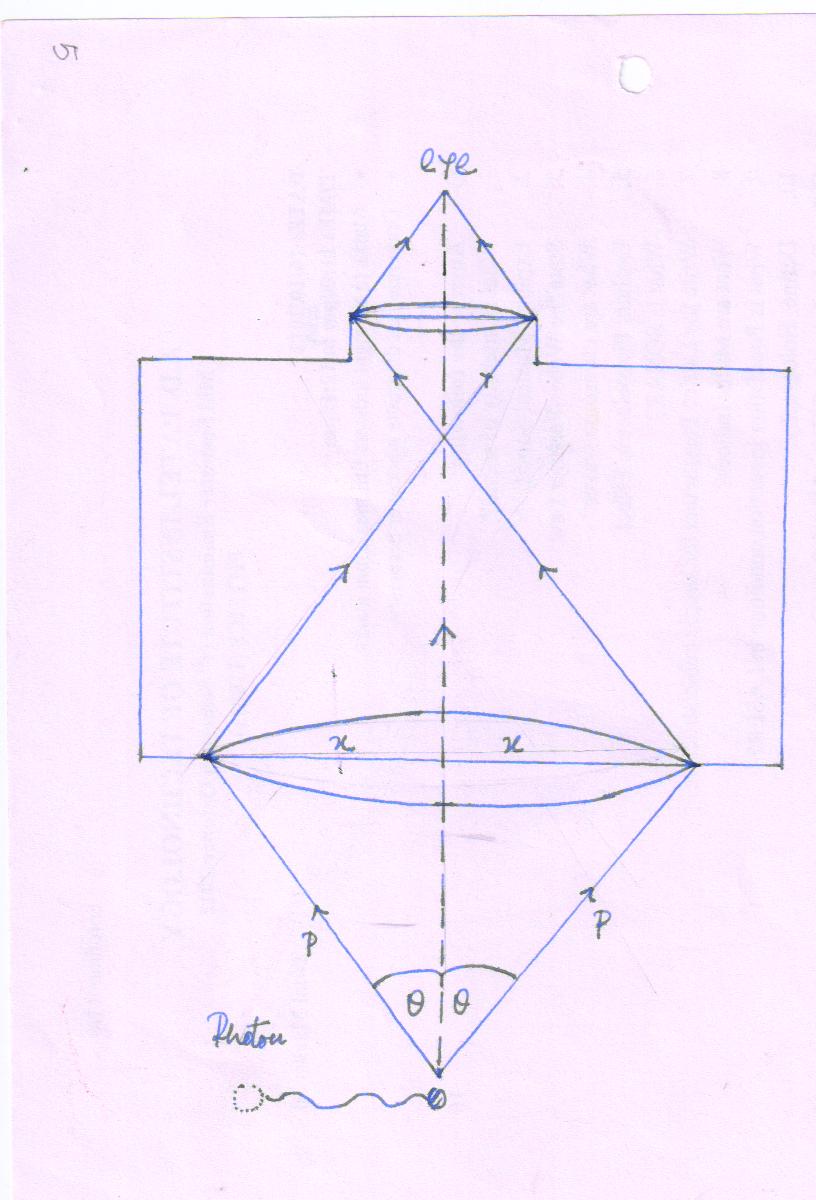
When a photon of initial momentum ρ = \[{h \over \lambda }\] after scattering (as a momentum is conserved in the collision) a particle enters in the field of view of microscope. A particle may be anywhere within angle 2\[\theta\]
Therefore, the momentum(x-component) in microscopic region is p sin \[\theta\] and -p sin \[\theta\]
Hence,
Δp = p sin \[\theta\] - (-p sin \[\theta\] )
Δp = 2p sin \[\theta\]
But , p = \[{h \over \lambda }\]
Δp = \[{{2h\sin\theta}\over\lambda}\]……..(2)
From (1) and (2)
Δx Δp = h
Product of uncertainties in position and momentum is of order of Plank’s Constant.
(2) Diffraction by single slit
Suppose a narrow beam of electrons passes through a single narrow slit (Δy) and produces a diffraction pattern on the screen.
Hence diffraction equation
\[d\sin \theta=n\lambda\]
For 1st order
\[d\sin\theta=\lambda\]
d - Width of the slit = Δy
Δy sin \[\theta\] =\[\lambda\]
Regarding diffraction pattern of on screen, all electrons have passed through the slit. But we can’t say that exact position of a particle within slit
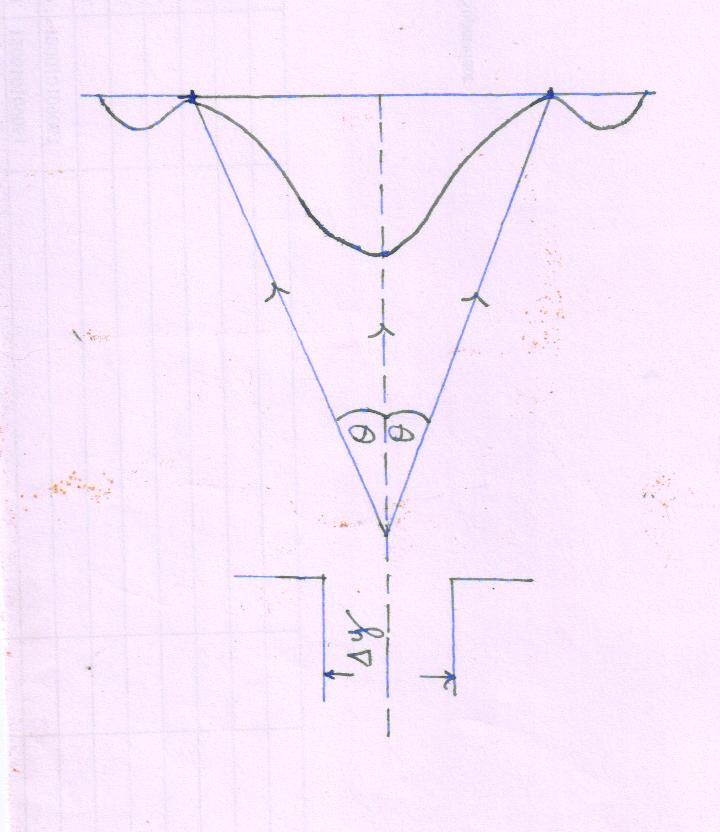
Hence the uncertainty can be determine the position of the electron equal to the width Δy.
Δy =\[{\lambda \over{\sin \theta }}\]
Here, initially electrons are moving along -axis and they have no component of momentum along -axis. After diffraction at the slit electrons are deviated from their initial path to form diffraction pattern and have a component . The y - component of momentum may lie between p sin \[\theta\] and -p sin\[\theta\].
The uncertainties in y – component of momentum = Δp = 2p sin\[\theta\]
But, p = \[{h \over \lambda }\]
Δp = 2p sin\[\theta\] = \[{{2h\sin\theta}\over\lambda }\]
Δy Δp = \[{\lambda\over{\sin \theta }}\] X \[{{2h\sin\theta }\over\lambda }\]
Δy Δp = 2h
Or
Δy Δp h
It shows the product of uncertainties in position and momentum is of order of Plank’s constant.