Site pages
Current course
Participants
General
MODULE 1. Magnetism
MODULE 2. Particle Physics
MODULE 3. Modern Physics
MODULE 4. Semicoductor Physics
MODULE 5. Superconductivty
MODULE 6. Optics
LESSON 24. High Temperature SuperConductor (HTS)
Introduction to High \[{T_c}\] superconductors
Basic concept of High Temperature Superconductors (HTS)
-
High-temperature superconductors are materials that behave as superconductors at unusually high temperatures.
-
Symbolically, it is denoted by high- \[{T_c}\] or HTS.
-
The first high- \[{T_c}\] superconductor was discovered in 1986 by the researchers Karl Müller and Johannes Bednorz.
-
The “ordinary” or “metallic” superconductors usually have transition temperatures (temperatures below which they exhibit the superconductivity phenomena) below 30 K (-243.2°C ).
-
The HTS have been observed with transition temperatures as high as 138 K (-135°C ).
-
The compounds of Copper and Oxygen (so-called "cuprates") were believed to have HTS properties, and the term high-temperature superconductor was used interchangeably with cuprites superconductor for compounds such as Bismuth Strontium Calcium Copper Oxide (BSCCO) and Yttrium Barium Copper Oxide (YBCO).
-
However, several iron-based compounds are now known to be superconducting at high temperatures.
History
The phenomenon of superconductivity was discovered by Kamerlingh Onnes in 1911, in metallic Mercury below 4 K (-269.15°C ). For seventy-five years after that, researchers attempted to observe superconductivity at higher and higher temperatures.
In 970, superconductivity was observed in certain metal oxides at temperatures as high as 13 K (-260.2°C ), which were much higher than those for elemental metals.
In 1987, K Alex Müller and J. Georg Bednorz were exploring a new class of ceramics for superconductivity. Bednorz encountered a Barium-doped compound of Lanthanum and Copper oxide whose resistance dropped down to zero at a temperature around 35 K (-238.2°C).
The superconductor with the highest transition temperature that has been confirmed by more than one independent research groups is mercury barium calcium copper oxide ( \[HgB{a_2}C{a_2}C{u_3}{O_8}\] ) at around 133 K.
Crystal structures of high-temperature ceramic superconductors
- The structure of high- \[{T_c}\] Copper oxide or cuprate superconductors are often closely related to perovskite structure [A perovskite structure is any material with the same type of crystal structure as Calcium Titanium Oxide ( \[CaTi{O_3}\] )].
- One of the properties of the crystal structure of oxide superconductors is an alternating multi-layer of \[Cu{O_2}\] planes with superconductivity taking place between these layers.
- This structure causes a large anisotropy in normal conducting and superconducting properties, since electrical currents are carried by holes induced in the oxygen sites of the \[Cu{O_2}\] sheets.
\[YBaCuO\] Superconductors Fig 15(1)
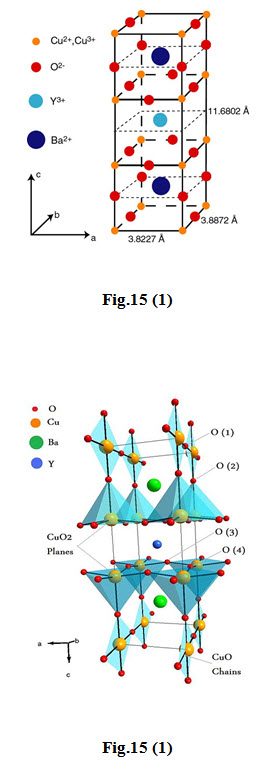
The first superconductor found with \[{T_c}\] > 77 K (liquid nitrogen boiling point) is Yttrium Barium Copper Oxide ( \[YB{a_2}C{u_3}{O_{7 - x}}\] )
The proportions of the 3 different metals in the \[YB{a_2}C{u_3}{O_{7}}\] superconductor are in the mole ratio of 1:2:3 for yttrium to barium to copper, respectively. So it is referred to as the 123 superconductor.
The unit cell of \[YB{a_2}C{u_3}{O_{7}}\] consists of three pseudocubic elementary perovskite unit cells.
Each perovskite unit cell contains a Y or Ba atom at the center: Ba in the bottom unit cell, Y in the middle one, and Ba in the top unit cell.
Thus, Y and Ba a are stacked in the sequence [Ba - Y - Ba ] along the c-axis.
All corner sites of the unit cell are occupied by Cu, which has two different coordination, Cu(1) and Cu(2) , with respect to oxygen.
There are four possible crystallographic sites for oxygen: O(1) , O(2) , O(3) and O(4) . The coordination polyhedral of Y and Ba with respect to oxygen is different.
The tripling of the perovskite unit cell leads to nine oxygen atoms, whereas \[YB{a_2}C{u_3}{O_7}\] has seven oxygen atoms and, therefore, is referred to as an oxygen-deficient perovskite structure. The structure has a stacking of different layers: \[\left( {CuO} \right)\left( {BaO} \right)\left( {Cu{O_2}} \right)\left( Y \right)\left( {Cu{O_2}} \right)\left( {BaO} \right)\left( {CuO} \right)\] .
One of the key features of the unit cell of \[YB{a_2}C{u_3}{O_{7 - x}}\] is the presence of two layers of \[Cu{O_2}\] . The role of the Y plane is to serve as a space between two \[Cu{O_2}\] planes.
In \[YBCO\] , the \[Cu\] - \[O\] chains are known to play an important role for superconductivity.
\[{T_c}\] is maximal near 92 K when \[x\] \[ \approx\] 0.15 and the structure is orthorhombic.
Superconductivity disappears at \[x\] \[ \approx\] 0.6, where the structural transformation of \[YBCO\] occurs from orthorhombic to tetragonal.
\[{B_i}\] - , \[Tl\] - and \[{H_g}\] - based high- \[{T_c}\] superconductors
-
The crystal structure of \[{B_i}\] - , \[Tl\] - and \[{H_g}\] - based high- \[{T_c}\] superconductors are very similar. Like \[YBCO\] ,
-
It is perovskite-type feature and the presence of \[Cu{O_2}\] layers also exists in these superconductors.
-
However, unlike \[YBCO\] , - \[Cu\] - \[O\] chains are not present in these superconductors. The superconductor has an orthorhombic structure, whereas the other high- superconductors have a tetragonal structure.
The \[B{i_2}\]-\[Sr\] -\[Ca\] -\[Cu\] -\[O\] -system has three superconducting phases forming a homologous series as \[B{i_2}S{r_2}C{a_{n - 1}}C{u_n}{O_{4 + 2n + x}}\] (n = 1, 2 and 3)
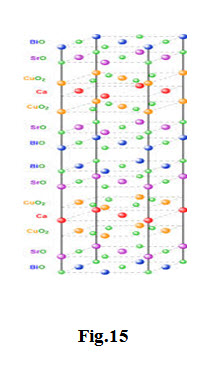
These three phases are \[Bi\]-2201, \[Bi\]-2212 and \[Bi\]-2223, having transition temperatures of 20, 85 and 110 K, respectively, where the numbering system represent number of atoms for \[Bi\], \[Si\] , \[Cu\] Ca and Cu respectively.
The two phases have a tetragonal structure which consists of two sheared crystallographic unit cells. The unit cell of these phases has double \[Bi - O\] planes which are stacked in a way that the Bi atom of one plane sits below the oxygen atom of the next consecutive plane. The Ca atom forms a layer within the interior of the \[Cu{O_2}\] layers in both \[Bi\]-2212 and \[Bi\]-2223; there is no \[Ca\] layer in the \[Bi\]-2201 phase. The three phases differ with each other in the number of \[Cu{O_2}\] planes; \[Bi\]-2201, \[Bi\]-2212 and \[Bi\]-2223 phases have one, two and three \[Cu{O_2}\] planes, respectively. The c axis of these phases increases with the number of \[Cu{O_2}\] planes (see table below). The coordination of the \[Cu\] atom is different in the three phases. The \[Cu\] atom forms an octahedral coordination with respect to oxygen atoms in the 2201 phase, whereas in 2212, the \[Cu\] atom is surrounded by five oxygen atoms in a pyramidal arrangement. In the 2223 structure, \[Cu\] have two co-ordinations with respect to oxygen: one \[Cu\] tom is bonded with four oxygen atoms in square planar configuration and another \[Cu\] atom is coordinated with five oxygen atoms in a pyramidal arrangement.
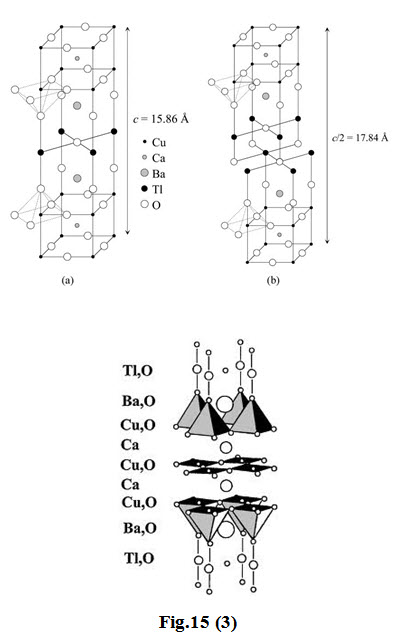
\[Tl - Ba - Ca - Cu - O\] Superconductor:
-
The first series of the \[TL\] -based superconductor containing one \[TL\]–\[O\] layer has the general formula \[TlB{a_2}C{a_{n - 1}}C{u_n}{O_{2n + 3}}\] whereas the second series containing two \[TL\] –\[O\] layers has a formula of \[T{l_2}B{a_2}C{a_{n - 1}}C{u_n}{O_{2n + 4}}\] with n = 1, 2 and 3
-
In the structure of \[T{l_2}B{a_2}Cu{O_6}\] ( \[TL\]-2201), there is one \[Cu{O_2}\] layer with the stacking sequence ( \[TL\]–\[O\] ) ( \[TL\]–\[O\] ) ( \[Ba\]–\[O\] ) ( \[Cu\]–\[O\] ) ( \[Ba\]–\[O\] ) ( \[TL\]–\[O\] )( \[TL\]–\[O\] ).
-
In \[T{l_2}B{a_2}CaC{u_2}{O_8}\] ( \[TL\]-2212), there are two \[Cu\]–\[O\] layers with a \[Ca\] layer in between. Similar to the \[T{l_2}B{a_2}Cu{O_6}\] structure, \[TL\]–\[O\] layers are present outside the \[Ba\]–\[O\] layers.
-
In \[T{l_2}B{a_2}C{a_2}C{u_3}{O_{10}}\] ( \[TL\]-2223), there are three \[Cu{O_2}\] layers enclosing \[Ca\] layers between each of these.
-
In \[TL\] -based superconductors, \[{T_c}\] is found to increase with the increase in \[Cu{O_2}\] layers. However, the value of \[{T_c}\] decreases after four layers in \[TlB{a_2}C{a_{n - 1}}C{u_n}{O_{2n + 3}}\] and in the \[TlB{a_2}C{a_{n - 1}}C{u_n}{O_{2n + 4}}\] compound, it decreases after three \[Cu{O_2}\] layers.
\[Hg - Ba - Ca - Cu - O\] Superconductor:
-
The crystal structure of \[HgB{a_2}Cu{O_4}\] ( \[Hg\] -1201), \[HgB{a_2}CaC{u_2}{O_6}\] ( \[Hg\] -1212) and \[HgB{a_2}C{a_2}C{u_3}{O_8}\] ( \[Hg\] -1223) is similar to that of \[TL\]-1201, \[TL\]-1212 and \[TL\]-1223, with in place of \[TL\] .
-
It is noteworthy that the \[{T_c}\] of the Hg compound (\[Hg\]-1201) containing one \[Cu{O_2}\] layer is much larger as compared to the one- \[Cu{O_2}\] -layer compound of thallium (\[TL\]-1201).
-
In the \[Hg\] -based superconductor, \[{T_c}\] is also found to increase as the \[Cu{O_2}\] layer increases.
-
For \[Hg\]-1201, \[Hg\]-1212 and \[Hg\]-1223, the values of \[{T_c}\] are 94, 128 and the record value at ambient pressure 134 K, respectively.
Table-1 High temperature superconductors
|
Formula |
Notation |
Tc (K) |
No. of Cu-O planes |
Crystal structure |
|
\[YB{a_2}C{u_3}{O_7}\] |
123 |
92 |
2 |
|
|
\[B{i_2}S{r_2}Cu{O_6}\] |
Bi-2201 |
20 |
1 |
|
|
\[B{i_2}S{r_2}CaC{u_2}{O_8}\] |
Bi-2212 |
85 |
2 |
Tetragonal |
|
\[B{i_2}S{r_2}C{a_2}C{u_3}{O_6}\] |
Bi-2223 |
110 |
3 |
Tetragonal |
|
\[T{l_2}B{a_2}Ca{O_6}\] |
Tl-2201 |
80 |
1 |
Tetragonal |
|
\[T{l_2}B{a_2}CaC{u_2}{O_8}\] |
Tl-2212 |
108 |
2 |
Tetragonal |
|
\[T{l_2}B{a_2}C{a_2}C{u_3}{O_{10}}\] |
Tl-2223 |
125 |
3 |
Tetragonal |
|
\[TlB{a_2}C{a_3}C{u_4}{O_{11}}\] |
Tl-1234 |
122 |
4 |
Tetragonal |
|
\[HgB{a_2}Cu{O_4}\] |
Hg-1201 |
94 |
1 |
Tetragonal |
|
\[HgB{a_2}CaC{u_2}{O_6}\] |
Hg-1212 |
128 |
2 |
Tetragonal |
|
\[HgB{a_2}C{a_2}C{u_3}{O_8}\] |
Hg-1223 |
134 |
3 |
Tetragonal |
Properties of High Temperature Superconductors.
-
HTS are brittle in nature
-
The properties of the normal state of these materials are highly anisotropic.
-
The Hall Coefficient is positive indicating the charge carriers are hole.
-
Their behavior can’t be explained by BCS theory.
-
The isotopic effect is almost absent in these materials.
-
The magnetic properties of these materials are highly anisotropic.
-
The effect of pressure is different on different materials e.g the application of pressure increases the critical temperature of \[LBCO\] compounds but decreases the critical temperature of \[YBCO\]
Application of HTSC
Commercial quantities of HTSC wire based on \[BSCCO\] are now available and applications include transformers, fault current limiters, power storage, motors and fusion reactors etc.
HTSC cables-
Lower voltage at the same power rating
Low impedance power guiding
Lower life cycle costs
No soil heating
No electromagnetic spray fields.
Electrical Machines-
High quality of generated power
Improved voltage stability
More reactive power is available at a given rating
Low total harmonic distortion
Low maintenance
Size and Weight reduction
High efficiency and low operating cost
Fault current limiters-
Over 100times faster response time
Time adjust response function
Full power protection cycles
Magnets-
Higher operational temperature range up to 77 K
Compact and high weight
Higher magnetic field of the order of 100 Tesla
Greater stability and thermal efficiency
Less complex cryogenic system
Higher signal to noise ratio
Transformers-
- HTS turns power transformer into compact, highly efficient and environment friendly performers.
RF and Microwave Filters-
Medical Diagnosis-