Site pages
Current course
Participants
General
Module 1. Basic Concepts, Conductive Heat Transfer...
Module 2. Convection
Module 3. Radiation
Module 4. Heat Exchangers
Module 5. Mass Transfer
Lesson-31 Introduction, Fick’s law of Diffusion, Mass Transfer Coefficients
Mass transfer plays an important and significant role in our daily lives. Mass transfer is a phenomenon that takes place in numerous activities which we undertake during day to day working such as adding sugar to tea, adding salt in a vegetable curry, evaporation of water in to air in a cooler, drying of wet clothes, etc.
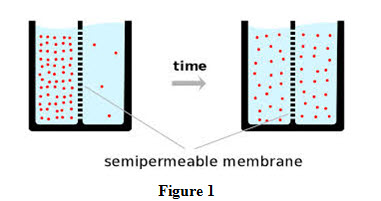
Mass transfer involves movement of matter of a substance from one place to another place. It is different from movement of bulk fluid such as air movement caused by a fan or blower and flow of water caused through a pipe due to pressure difference or by a pump. In mass transfer, movement is caused by differences in concentration of the substances between two regions. Mixing of two gases upon removal of a boundary separating them in a container is an example of mass transfer on account of concentration differences as shown in Figure 1.
Concentration of a substance quantifies the amount of the substance per unit volume. This amount can be on a mass or molar basis. The mass concentration of a particular substance called the density and is expressed as

In mass transfer concentration means molar concentration and is expressed as

In mass transfer, movement of a matter of substance occurs due to concentration gradient and movement is always from high concentration region to low concentration region. The mass transfer will continue till the concentration differences between two regions exist and will stop when equilibrium is obtained. Mass transfer basically deals with transport of species:
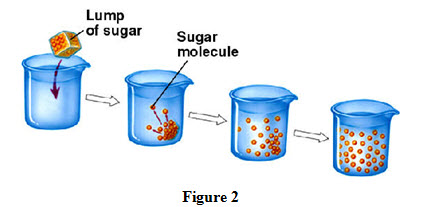
• within a medium for example sugar dissolves in a cup of tea to sweeten the entire tea cup as shown in Figure 2.
• across an interface for example from one medium to another i.e. spreading of food odour in the entire house as shown in Figure 3.
Mass transfer is similar to heat transfer in following ways:
The driving force for heat transfer is temperature gradient whereas mass transfer occurs due to concentration gradient.
Heat transfer always takes place from high to low temperature regions, similarly mass is transferred towards low concentration regions, thereby, decreasing the temperature gradient.
Heat transfer stops immediately when temperature difference becomes zero, similarly, mass transfer ceases when concentration gradient is reduced to zero.
The rates of heat and mass transfer depend upon the driving potential and resistance.
Types of Mass Transfer:
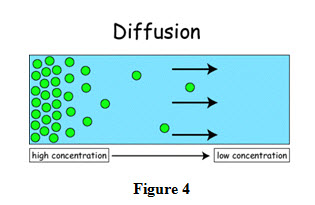
Transfer of mass takes place under different conditions and depending upon the conditions, it can classified in to different categories which are shown in Figure 4.
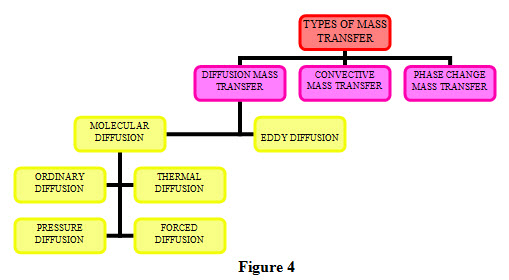
1. Diffusion Mass Transfer: Diffusion mass transfer can be classified two categories:
i) Molecular Mass Diffusion: This type of mass transfer occurs at macroscopic level in which transfer of mass takes place from a region of high concentration to low concentration in a mixture of liquids or gases. Transfer of mass by diffusion occurs due to
Presence of concentration gradient in a mixture and is called ordinary diffusion as shown in Figure 4.
Presence of thermal gradient and is termed as thermal diffusion
Presence of pressure gradient and is termed as pressure diffusion
Presence of external forces and is termed as forced diffusion
ii) Eddy Diffusion: Mass transfer by eddy diffusion occurs when one of the diffusing fluids is in turbulent motion and is in addition to the diffusion mass transfer. The turbulent motion increases mass transfer.
2. Convective Mass Transfer: Mass transfer occurring between a moving fluid and a surface or between two relatively immiscible fluids is termed as convective mass transfer.
3. Mass Transfer by Phase Change: This type of mass transfer occurs due to change in the phase of a substance.
Fick’s Law of Diffusion:
The diffusion process is governed by mass transfer laws which are very similar to heat transfer laws and govern the relationship between mass flux and concentration gradient. The basic law of diffusion was proposed in 1855 by Adolf Fick which is expressed as
Mass Flux = Constant of Proportionality X Concentration Gradient (1)
Consider a system in which a partition separates two gases B and C and the two gases are maintained at same temperature and pressure initially as shown in Figure 5.
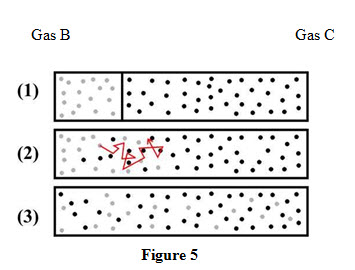
On removal of the partition, molecules of gas B move towards right where concentration is low while molecules of gas C move towards low concentration area i.e towards left. The molecules of both the gases diffuse with each other gradually. The diffusion rate is given by Fick’s law and is expressed as

mb/A is Mass flux or mass flow per unit area, Kgm /hr-m2
A is area through which mass is flowing, m2
Cb is mass concentration or molecules per unit volume, kg/m3
![]() is concentration gradient for gas B
is concentration gradient for gas B
Dbc is the diffusion coefficient or mass diffusivity, m2/Sec
The unit of diffusion coefficient or mass diffusivity is same as the units of thermal diffusivity and kinematic viscosity which is also called momentum diffusivity. Diffusion coefficients are generally determined experimentally and increase with increase in temperature but decrease with increase in pressure.
Mass flux for gas C can be expressed as

By using characteristic gas equation, Fick’s Law can be expressed in terms of partial pressure.
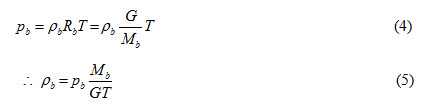
Density represents mass concentration to be used in Fick’s Law. Therefore,

Substituting the value of Cb from equation (6) in to equations (2) and (3), we get

The above equations hold good for isothermal diffusion only.
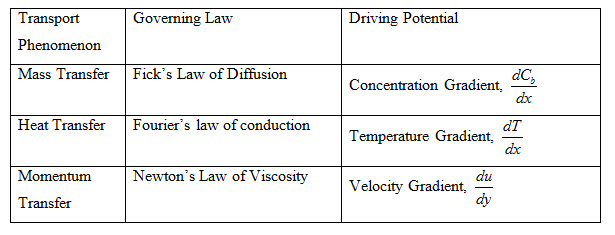
The Fick’s law of diffusion as given by equations (2) and (3) is similar to Fourier’s law of heat conduction and Newton’s law of viscosity expressed by equations (9) and (10) respectively.

Analogy between mass, heat and momentum transfer has been shown in Table 1
Table 1:
Some important aspects of Fick’s law of diffusion are summarized below;
Fick’s law is applicable for all matter irrespective of it state: solid, liquid or gas. Mass transfer is inversely proportional to molecular spacing.
Similar to heat transfer which occurs in direction of decreasing temperature, mass transfer by diffusion also occurs in the direction of decreasing concentration.
Apart from concentration gradient, mass transfer can occur due to presence of temperature gradient, pressure gradient or external force. However, Fick’s law gives rate of mass transfer on account of concentration gradient only and effect of other parameters is considered to be small or negligible.
Diffusion coefficients in gases are highest, followed by liquids and solids.
Diffusion coefficient or mass diffusivity is a function of temperature, pressure and composition of a system. However, for ideal gases and dilute liquids, diffusion coefficient depends on temperature and pressure and is independent of system composition.