Site pages
Current course
Participants
General
MODULE 1. Introduction to production of agricultur...
MODULE 2. Advance in material for tractor and agri...
MODULE 3. Advanced manufacturing techniques
MODULE 4. Heat treatment of steel
MODULE 5. Industrial lay out planning and quality ...
MODULE 6. Economics of process
MODULE 7. Techno economic feasibility of project r...
MODULE 8. Servo motors, drives and controllers
MODULE 9. CNC controllers for machine tools
MODULE 10. CNC programming
MODULE 11. Assembly and plant automation storage a...
LESSON 16. MICRO STRUCTURE ANALYSIS OF METALS
4.1 Introduction
The engineering metals always play an important role in an industry, because the process of all manufacturing starts with the raw material. These are broadly divided into two groups,
i.e., metals and non metals. A metal may be described as a material which is solid at room temperature, has relatively high density, high, melting temperature, low specific heat, good electrical and thermal conductivity, strength, hardness, etc. Most metals are elastic to a limit: they deform in proportion to stress and return to the original state when the stress is released. At higher stresses they deform plastically. Some metal will accept a deal of plastic deformation before failure.
A metal can exist in the gaseous, liquid and solid state. The state depends on the pressure exerted on the metal and on its temperature. A molecule is the smallest particle of an element or compound that can stay independently. A molecule that consists of one atom is called a monoatomic (Ex. rare gases). Diatomic molecules are those which are made of two atoms (Ex.hydrogen, nitrogen, oxygen). Polyatomic molecules are those which contain more than two atoms; phosphorus molecules contain four atoms.
Atom: It may be defined as smallest particular that retains the physical characteristics of that element. Atoms occupy fixed positions and strong bonds exist between them. Atoms are located at sites, which are divided by the structure of the atoms and nature of bonds. Combination of properties of atom and the way in which they are assembled determines the mechanical properties of particular metal.
Matter: May be defined as a collection of atoms whose position and behavior determines its properties and characteristics.
The materials chiefly used in the shops are metals which may be broadly divided into the following two groups.
Ferrous metals: The metals which contain iron as their chief constituent are called ferrous metals. The various ferrous metals used in industry are pig iron, cast iron, wrought iron, steel etc.
Non-ferrous metals: The metals which contain a metal other than iron as their chief constituent are called non-ferrous metals. The various nonferrous metals used in industry are aluminium, copper, zinc, leaf, brass, tin etc.
The non-metals in the solid state are mostly brittle and poor electrical and thermal conductors. They do not form alloy but combine chemically to form compound. Some of the non-metals are glass, plastics, wood, concrete etc.
4.2. Structure of solids
There are two types of solids. They are crystalline and amorphous. All solid substance is either amorphous solids or crystalline solids.
4.2.1. Amorphous solids
In the amorphous solids, the atoms are arranged chaotically, i.e., the atoms are not arranged in a systematic order. Amorphous solids are solids with random unoriented molecules. Amorphous solid substance does not possess long-range order of atoms positions. The common solids are wood, plastics, glass, paper, rubber etc.
4.2.1.2 Crystalline solids
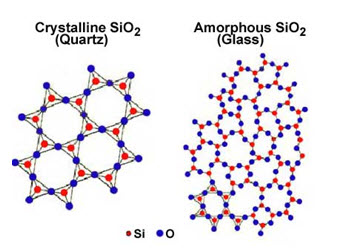
In crystalline solids, the atoms making up the crystals arrange themselves in a definite and orderly manner and form. Crystalline solids are arranged in fixed geometric patterns or lattices. All solid metals such as iron, copper, aluminium etc are crystalline solids. The arrangement of atoms in the amorphous solids or crystalline solids is shown in Fig.4.1 below.
Fig. 4.1 Structure of solids
4.3. Metals crystal structure
A crystal structure is a unique arrangement of atoms in a crystal. A crystal is a periodic array of atoms. The crystal structure of a material or the arrangement of atoms in a crystal can be described in terms of its unit cell. A crystal is composed of unit cells. A unit cells contains the smallest number of atoms, which when taken together have all properties of the crystal of the particular metal. The lengths of the edges of a unit cell and the angles between them are called the lattice parameters.
Crystals are conceptually built up from unit cells. The Unit cell is the basic building block for a crystal. The unit cell is defined as the smallest parallelepiped which could be transposed in three coordinate directions to build the space lattice. The space lattice of various substances differ in the size and shape of their unit cells. A crystal is basically a whole bunch of unit cells stacked end to end in 3D. Six parameters describe the shape of the unit cell - the length of the unit cell edges (a,b,c) and the angles between them (alpha, beta, gamma). The angle alpha (α) is the angle between the b and c cell edges, beta (β) between a and c, gamma (γ) between a and b. a, b, c represent the vectors of the edges of the unit cell, in whatever coordinate system it is used. Minerals belonging to the same crystal system have the same shaped unit cell The length of the edges of the unit cell vary depending upon the types of atoms and how they are arranged. The unit cell is given by its lattice parameters, the length of the cell edges and the angles between them, while the positions of the atoms inside the unit cell are described by the set of atomic positions (xi,yi,zi) measured from a lattice point.
Fig.4.2 shows the unit cell of a three dimensional crystal lattice. It is formed by the primitives or intercepts a, b, and c along the three axes respectively. The three angles are called (alpha, beta, gamma) are called interfacial angles. Both the intercepts and interfacial angles constitute the lattice parameters of the unit cell.
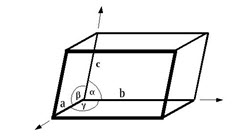
Fig.4.2. Lattice arrangement of a unit cell.
4.4. Crystal system
The crystal systems are a grouping of crystal structures according to the axial system used to describe their lattice. Each crystal system consists of a set of three axes in a particular geometrical arrangement. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The seven basic crystal systems are cubic, tetragonal, hexagonal, orthorhombic, rhombohedral, monoclinic and triclinic.
4.5. Crystal lattice
The definite and orderly manner and form of the atoms producing a small, repeating, three dimensional, geometrical pattern in aggregate is called crystal lattice or space lattice. The following three types of crystal lattice are usually found in most of the metals. There are fourteen possible types of space lattices in these seven crystal systems. But the following three types are usually found in most of the metals.
4.5.1. Body centered cubic (BCC) space lattice
In body centered cubic space lattice, there are nine atoms. The eight atoms are located at the corners of the cube and one atom at it centre. This type of lattice is found in alpha iron, tungsten, chromium, manganese, molybdenum, tantalum, barium, vanadium etc.,
4.5.2. Face centered cubic (FCC) space lattice
In face centered cubic space lattice, there are fourteen atoms. The eight atoms are located at the corners of the cube and six atoms at the centers of six faces. This type of lattice is found in gamma iron, aluminum, copper, lead, silver, nickel, gold, platinum, calcium etc.,
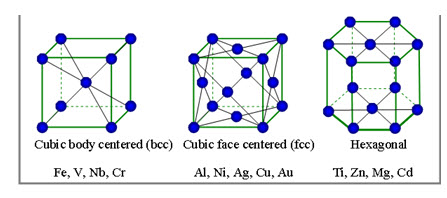
Fig. 4.3. Crystal lattice
4.5.3. Closed packed hexagonal (CPH) space lattice
In close packed hexagonal space lattice, there are seventeen atoms. The twelve atoms are located at the twelve corners of the hexagonal prism, one atom at the centre of each of the two hexagonal faces and three atoms are symmetrically arranged in the body of the cell. This type of lattice is found in zinc, magnesium, cobalt, cadmium, antimony, bismuth, beryllium, titanium, zirconium etc.,
4.6. Formation of grains.
Grain is a small region of a metal, having a given and continuous crystal lattice orientation. Each grain represents small single crystal. Grains form as a result of solidification or other phase transformation processes. Grains shape and size change in course of thermal treatment processes. The normal grain size varies between 1µm to 1000 µm. Grain structure of a solid is an arrangement of differently oriented grains, surrounded by grain boundaries. All metals are crystalline and crystals are made up of several atoms. Grains are formed when he liquid metal solidifies. The process of solidification or crystallization will proceed if the liquid is cooled below the equilibrium temperature (tempo at which the given metal exists simultaneously in a liquid and solid phase) of solidification.
The solidification commences by the formation of small nuclei scattered at random in the cooling liquid. At these points, a few atom assume an orderly arrangement to give the unit cubic structure and growth takes place in three dimensions. The individual crystal or gains are aggregated to form the visible mass of a solid metal. Solidification or freezing is started when two or more atoms associate themselves to form a very small crystal, called nuclei. This may happen simultaneously at a number of locations throughout the liquid metal. This means that a number of nuclei form within the solidifying metal as it continuous to cool. The small crystal or the nucleus develops in the freezing of a liquid metal in the form of dendrites (dendron is a Greek word meaning a tree). A dendrite consisting of unit cells, which are then formed on the original straight line and so on as shown in fig. First long branches known as primary axis of the dendrite is formed and then the branches evolve from their edges and grow as secondary axis. Lastly, the tennary axis evolve and grow on secondary axis. This action is called nucleation or crystal growth and continues until all the metal between the branches has solidified.
Grain - A solid polyhedral (or many sided crystal) consisting of groups of atoms bound together in a regular geometric pattern
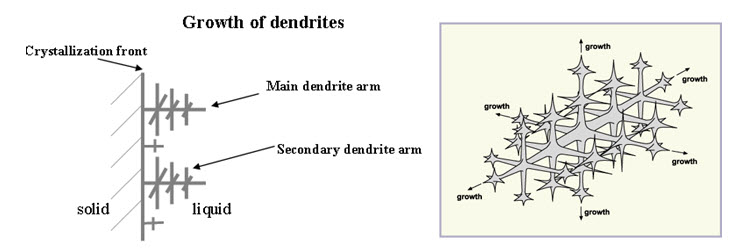
Fig. 4.4. Growth of a metal dendrite
As the dendrite grows, the spaces between its arms fill up. Outward growth stops when growing arms meet others. Eventually the entire liquid solidifies, and there is little trace of the original dendritic structure, only the grain into which the dendrites have grown.
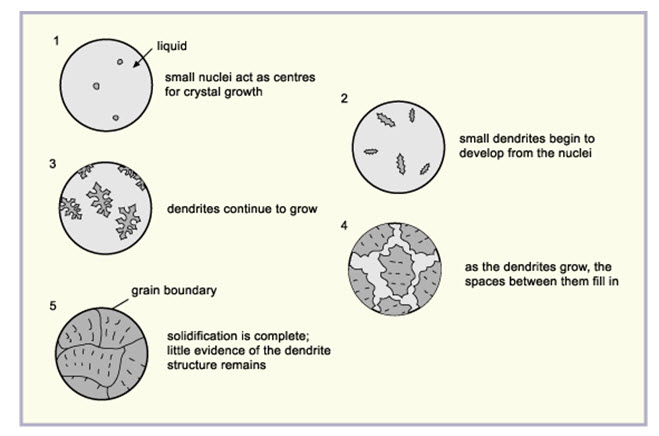
Fig. 4.5. Solidification of a metal
Each crystal of proper geometrical form but having different orientation grows outward until it comes in contact with the adjacent crystal as depicted in Fig.4.4. The crystal growth is then gradually distorted due to interference of each crystal within its neighbors. Consequently, the crystals observed in the fracture of a metal of irregular shape are called grains. The final solid is said to be polycrystalline because of many crystals or grains of different orientations exist init. Within each crystal the atoms may be “packed together “ in geometrical pattern. Grain is a small region of a metal, having a given and continuous crystal lattice orientation. Each grain represents small single crystal.
A boundary known as grain boundary is formed between two adjacent crystalline growths because of different orientations of the grains. If the orientations of the grains would not differ, they would unite, and there would not have any grain boundary. The crystals outward growth is stopped by impingement on adjacent crystals. The contact surfaces formed by adjacent crystals (because of orientation other grains) are called grain boundary. Grain boundaries interrupt the continuity of the lattice planes and increase the resistance of the metal to cold deformation. A grain boundary is the interface between two grains in a polycrystalline material. The grain boundary refers to the outside area of a grain that separates it from the other grains.
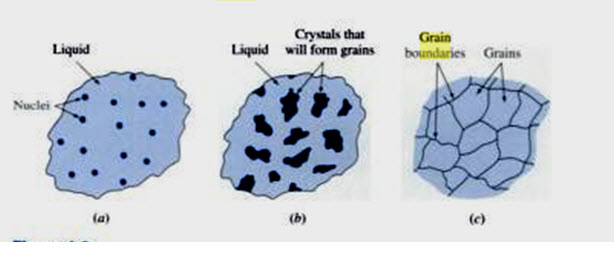
Fig. 4.6. Stages in solidification of metals
(a) Formation of nuclei (b) Growth of nuclei into crystal; (c) Joining together of crystals to form grains and associated grain boundaries
Each crystal or the grain is built up of thousands of small unit cells or cubes. The axes of the cubes all point in the same direction, but this direction vary from one crystal to another. This effect is called as orientation of the atoms. The orientation of the atomic pattern in a given crystal but varies from grain to grain. The grain size has an important effect on the mechanical properties of metal such as strength, ductility, hardness etc.