Site pages
Current course
Participants
General
MODULE 1. Planning of Farmstead
MODULE 2. Physiological Reactions of Livestock
MODULE 3. BIS Standards
MODULE 4. Farm Structures
MODULE 5.
MODULE 6. Rural Living and Development
MODULE 7. Water Supply
Topic 8
Topic 9
Topic 10
LESSON 16. Sources of Water Supply
Introduction
For any living being water, air, food, shelter, etc, are primary needs. Of these needs, water is the most important need. Water is used for various purposes like
-
Drinking & Cooking.
-
Bathing and washing
-
Watering of lawns & garden.
-
Heating and air-conditioning systems.
-
Growing of crops.
-
Street washing
-
Fire fighting
-
Recreation in swimming pools, fountains & case.
-
Steam power & various industrial processes.
Quantity of water:
In order to design the water supply scheme in a town or city, it is necessary to find out the total of water required. Therefore the demand of the town has to be determined. Then a suitable water supply scheme is to be found out to meet net water demand. But it is difficult to determine the actual demand to the variable factors affecting it. Hence a thumb rule or an example formula is issued to assess the demand of the town.
The various types of demands of a city or town are
-
Domestic water demand
-
Commercial and industrial demand.
-
Fire-demand.
-
Demand for public Use.
-
Compensate losses.
i) Domestic water demand:
It includes the quantity of water required in the houses for drinking, bathing, cooking, washing, etc. This demand mainly depends upon the habits, serial status, climatic conditions and customs of the people. In India, on an average, the domestic consumption of water under normal conditions is @ 135 ht/day/capita. But in developed countries the figure may go upto 350 lit/day/captia. This 135 liters will cater the following needs.
Drinking - 5 lit
Cooking - 5 lit
Bathing - 55 lit
Clothes washing - 20 lit
Utensils washing - 10 lit
House waster - 10 lit
Flushing of latrines - 30 lit
Total - 135 lit/day/capita
ii) Commercial and Industrial Demand:
Various commercial buildings and commercial centres are office buildings, warehouses, stores, hotels, shopping centres, health centres, schools, temples, cinema houses, railway & bus stations. The water requirement of commercial and public places varies between 4.5 lit/day/capita to 450 lit/day/capita. An average of 20-25% of total water consumption may be considered for the design purpose.
iii) Fire Demand:
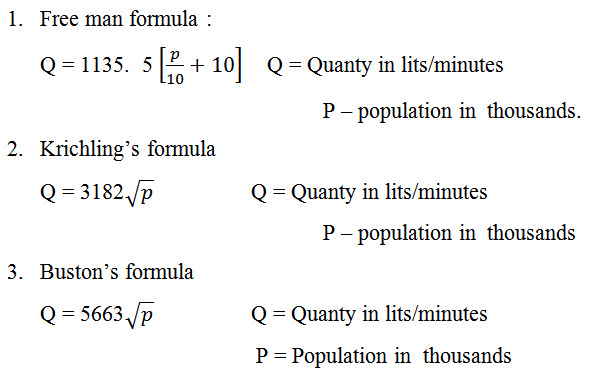
During outbreak of fine in a city, of water is required for throwing it over the fire extinguish it. For this purpose, or provision should be made in the water work to supply sufficient quantity of water. The quantity of water required for fine fighting is given the following empirical formula. National Board of Fire underwriters Formula is given by,
![]()
Where Q = Quantity of water required in lit
P = population of the town in thousands
This formula is used when population is upto 2l.
iv) Demand for public Use:
This demand include, water required for washing & sprinkling on roads, cleaning of Watering of parks, garden, foundation etc.
Sources of water can be broadly classified as follows:
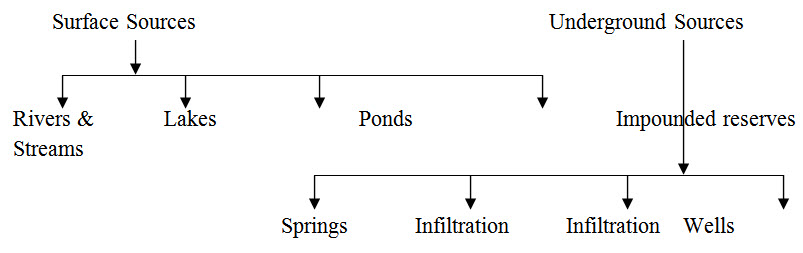
Surface Sources
The surface sources are rivers, lakes, ponds & impounded reservoirs. The water yield of the surface sources vary from season to season.
Streams
In mountainous region streams are formed by the runoff. Small streams are useful as a source of water, only for small villages. Large and perennial stream may be used as sources of water supplied by providing storage reservoirs across them. The streams feed their water to the lakes or rivers.
Rivers
They are the most important sources of water for public water supply schemes. Rivers may be perennial or non-perennial. Perennial rivers have water throughout the year. They are fed by rains during rainy season and by snow during summer season.
QUALITY OF WATER
Absolutely pure water is never found in nature. It is 2 molecules of hydrogen and 1 molecule of oxygen. Water may contain impurities, some of which are harmful for consumption while some of them are useful for public use. Hence, the impurities should be removed only upto certain extent so that, it may not be harmful to public health. So, a water which is not chemically pure but does not contain anything harmful to human health is called ‘Wholesome water’.
The impurities of water can be listed broadly as follows :
1) Suspended impurities
These impurities are solid particles that are large enough to be removed by filtration or if they are heavier they will settle down. The suspended impurities are macroscopic in size and cause turbidity in water. Suspended impurities are algae, fungi, protozoa, bacteria, clay silt, etc.
2) Dissolved impurities
Some impurities in the form of solid, liquid & gas are dissolved in water when it moves over the rocks, soils etc. These may contain Organic compounds, inorganic salts and gases etc. The concentration of total dissolved solids is expressed in ppm & is obtained by weighing the residue after evaporating the filtered water sample. They may be Ca, Mg, Na of HCO3, CO3, SO4, F, Cl2 metals & gases.
3) Organic impurities
Again these organic impurities may be either suspended organic impurities or dissolved organic impurities. Mostly all the colloidal impurities are associated with organic matter containing bacteria. These are the chief source of epidemics. These organic impurities are either suspended or dissolved vegetable or animal matters.
EFFECTS OF VARIOUS IMPURITIES
|
IMPURITIES |
EFFECT |
||
|
Suspended : |
Bacterias |
- |
Cause disease |
|
|
Algae, Protozoa |
- |
Cause odour, turbidity & colour |
|
|
Clay, Silt |
- |
Cause turbidity
|
|
Dissolved : |
Ca & Mg – Bicarbonate Carbonate Sulphate |
- - - |
Cause hardness, alkalinity & softening Cause hardness, alkalinity & softening Cause hardness
|
|
|
Na – Fluroides Chloride Manganese |
- -
|
Cause mottle enamel of teeth Imparts taste black or brown colour the water |
|
|
Iron oxide
Metals – Lead - Arsenic |
-
- - |
taste, corrosiveness, hardness & red colour. Lead poisoning Poisoning |
|
|
Gases - Oxygen Co2 – Carbon-di-oxide H2S - Hydrogen Sulphide |
- -
-
|
Corrode the metals Cause acidity & Corrode metal
Cause rotten egg odour, Corrode metal, acidity |
|
Organid Impurities: |
Suspended – Vegetable
Animal (live) Dissolved – Vegetable Animal (dead) |
-
- - |
Cause objectionable colour, taste & acidity. Produce harmful disease germs. Produce bacteria Cause pollution of water and produce disease germs. |