Site pages
Current course
Participants
General
Module 1. Moisture content and its determination.
Module 2. EMC
Module 3. Drying Theory and Mechanism of drying
Module 4. Air pressure within the grain bed, Shred...
Module 6. Study of different types of dryers- perf...
Module 5. Different methods of drying including pu...
Module 7. Study of drying and dehydration of agric...
Module 8. Types and causes of spoilage in storage.
Module 9. Storage of perishable products, function...
Module 10. Calculation of refrigeration load.
Module 11. Conditions for modified atmospheric sto...
Module 12. Storage of grains: destructive agents, ...
Module 13. Storage of cereal grains and their prod...
Module 14. Storage condition for various fruits an...
Module 15. Economics aspect of storage
Lesson.15 Osmotic Dehydration
Mechanism
In osmotic dehydration, the fruit or vegetable pieces are immersed in concentrated aqueous solution of high osmotic pressure (hypertonic media) for a specified time and temperature. The driving force for water removal is the concentration gradient between the solution and the intracellular fluid. If the membrane is perfectly semi permeable, solute is unable to diffuse through the membrane into the cells. Selective properties of cell membranes make it possible for water and low-molecular cell sap components diffuse into the surrounding solution of higher osmotic pressure. However, it is difficult to obtain a perfect semi permeable membrane in food systems due to their complex internal structure, and there is always some solid diffusion into the food, which means that osmotic dehydration, is actually combination of simultaneous water and solute diffusion process (Chaudhari et al., 1993;Ghosh et al., 2004).
In general, during osmotic pre-concentration, two major counter current flow take place simultaneously across the semi permeable cell membrane,(i) water diffusion out of the food into the solution, at a faster rate initially and slowly afterwards and, (ii) solute penetration in the opposite direction, at a slower rate initially but increasing with time (Chaudhari et al., 1993). A third transfer process, leaching of product solutes (sugars, acids, minerals, vitamins) into the medium, although recognized as affecting the organoleptic and nutritional characteristics of the product, is considered quantitatively negligible (Lazarides et al., 1995). Fig.1 shows the different flows, in and out of the fruit/vegetable tissue.
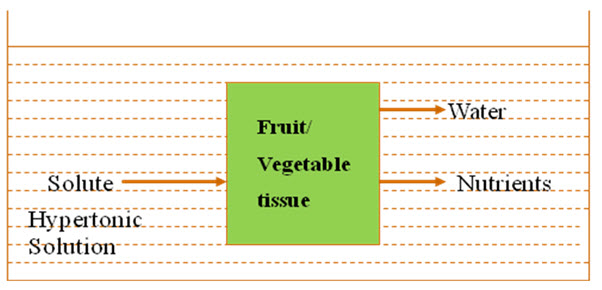
Fig.1. Mass transfer across a fruit/vegetable tissue during osmotic dehydration
Advantages:
The advantages of osmotic dehydration are as follows (Islam and Flink, 1982; Chaudhari et al., 1993; Ghosh et al., 2004).
-
Mild heat treatment favours less heat damage to colour and flavour of the product with superior sensory attributes.
-
The use of sugar or syrup as osmotic agent prevents much of the loss of flavour commonly found with ordinary air or vacuum drying.
-
Enzymatic and oxidative browning is prevented as the fruit pieces are surrounded by sugar, thus making it possible to retain good colour with little or no use of sulpher dioxide.
-
Energy consumption is much less as no phase change of moisture is involved during dehydration. Osmotic dehydration with syrup re-concentration demands two to three times less energy compared to convection hot air drying.
-
Acid removal and sugar uptake by the fruit pieces modify the composition (sugar to acid ratio) and improve the taste and acceptability of the final product.
-
It partially removes water and thus reduces water removal load at the dryer.
-
It increases solid density due to solid uptake and helps in getting quality product in freeze-drying.
-
If salt is used as an osmotic agent, higher moisture content is allowed at the end of the drying as salt uptake influences the water sorption behaviour of the product.
-
The final product shows much lower rehydration rate, lower hygroscopicity and better textural quality after rehydration in comparison to other dehydration techniques.
-
The storage life of the product is greatly enhanced.
-
Simple equipment is required for the process.
Disadvantages:
It also has some disadvantages (Chaudhari et al., 1993;Ghosh et al., 2004).
-
The reduction in acidity level reduces the characteristic taste of some products. This can be overcome by adding fruit acid in the solution.
-
Solute uptake and leaching of valuable product constituents often lead to substantial modification of the original product composition with a negative impact on sensory characteristics and nutritional profile.
-
Sugar coating is not desirable in certain products and quick rinsing may be necessary after the treatment.
-
Sugar uptake results in the development of a concentrated solids layer under the surface of the fruit, upsetting the osmotic pressure gradient across the fruit interface and decreasing the driving force for water flow.
-
In terms of final product characteristics, sugar uptake affects both rehydration and flavour retention due to lower rehydration of sugar in the fruit, compared with fruit tissue itself.
Osmotic dehydration process
Since osmotic dehydration generally will not give a product of low enough moisture content to be considered self-stable, it has to be coupled with other methods of drying, viz., hot air drying, vacuum drying, freeze drying etc (Ponting, 1973; Sagar, 2001). The schematic diagram of osmotic dehydration process is shown in Fig. 2. All the steps given in Fig. 2 may not be followed as such and are subjected to change considering the types of material being processed (Chaudhary et al., 1993). It is usually not worthwhile to use osmotic dehydration technique for more than 50% weight reduction because of the decrease in the osmosis rate with time (Chaudhary et al., 1993; Ghosh et al., 2004).
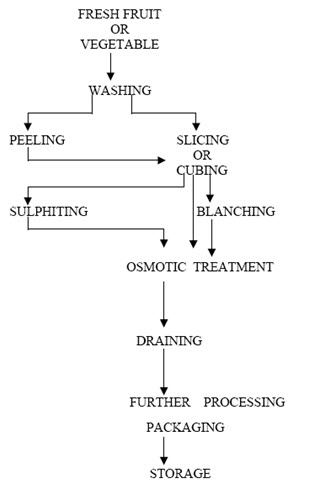
Fig.2.Osmotic dehydration process
Factors affecting osmotic dehydration process
Product characteristics
Species, Variety and Maturity Level
Not only different species, but also different varieties of the same species, even different maturity levels of the same variety have been found to give substantially different response to osmotic dehydration. Under identical conditions five varieties of apricots showed different mass reduction during osmotic dehydration.
Tissue Location
The tissue at different locations in the same fruit or vegetable responds differently to osmotic dehydration. The inner and outer parenchyma tissue of Grany Smith variety of apple showed different water loss and solid gain at same osmotic dehydration conditions (Marvroudis et al., 1998). The interconnectivity and pore spaces of two kinds of tissues show different mass transfer due to different pathways of transport.
Size and shape
The kinetics of osmotic dehydration is affected by the size and shape of the samples, due to different specific surface area or surface to thickness ratio. Also different forms of samples are selected on the basis of end-use of product after further processing (Islam and Flink, 1982; Lerici et al., 1985; Sankat et al., 1996;Ghosh et al., 2004).
Concentration of osmotic solution
The choice of the solute and its concentration depends upon several factors. The organoleptic evaluation of the final product is the most important consideration besides the cost of the solute. The solubility of the substance in water is crucial for its effect on maximum possible concentration in the osmotic solution. The capacity of the compound to lower the water activity will also affect the driving force responsible for the mass transport. It is also not desirable to have a solute that reacts with the final product. During osmosis, the kinetics of water removal, the solid gain and the equilibrium moisture content are strongly affected by the kind of osmotic agent, its molecular weight and ionic behaviour. Sucrose and sodium chloride are most commonly used osmotic agents. Sodium chloride is found to be an excellent agent for vegetables as it changes cell permeability but has limited use in fruits dehydration due to salty taste (Hawkes and Flink, 1978; Lerici et al., 1985 Pawar et al., 1988; Yang and Le Maguer, 1992; Erketin and Cakaloz, 1996; Sagar, 2001;Pokharkar, 2001).
The use of sucrose salt mixture as osmotic reagent also have beneficial effects as it develops high osmotic potential thereby causing higher water loss, retarding oxidative and non-enzymatic browning, and gives product with better quality (Islam and Flink; 1982).
Process temperature
Temperature of osmotic solution plays an important role in osmotic dehydration process. The effect of temperature is more pronounced between 30 to 60°C for fruits and vegetables on the kinetic rate of moisture loss without affecting solid gain (Ponting, 1973; Rastogi and Raghavarao, 1995; Pokharkar, 2001).
Sample to solution ratio
The sample weight to solution ratio is an important consideration during the osmosis. The change in ratio affects the mass transfer during osmosis up to a certain limit. Most of research workers used the sample to solution ratio ranging from 1:1 to 1:5 in order to study the mass transfer kinetics by following changes in concentration of solution and other factors (Islam and Flink, 1982; Lenart and Flink, 1984; Grabowski et al., 1994; Welti et al., 1995; Erketin et al., 1996; Pokharkar, 2001). Higher ratio (1:10 to 1: 60) can also be used in order to avoid significant dilution of the medium due to uptake of water from sample and loss of solute to the sample, and subsequent decrease in the osmotic driving force during the osmotic dehydration (Karthanos et al., 1995; Lazarides et al., 1995).
Method/system to enhance the mass transfer
Agitation during the osmotic dehydration reduces the mass transfer resistance at the surface of the fruit/vegetable sample and provides a uniform distribution of osmotic solution around the product. But gentle agitation has little effect on the osmosis rate (Erketin et al., 1996; Pokharkar and Prasad, 1997; Ghosh et al., 2004). Agitation may cause damage to the sample and may be difficult to apply.
Several methods and systems can be used to enhance the mass transfer during osmotic dehydration process. These methods include high electric field pulse treatment, centrifugal force, high hydrostatic pressure and application of vacuum. Some pretreatments like freezing, microwave treatment and blanching before osmotic dehydration can increase the rate of moisture loss and solid gain. The systems available for accelerating the mass transfer during osmotic dehydration are cylindrical vessel and impeller rotating at center, pipe holding food with flow of osmotic solution, horizontal cylindrical tank with a helical tube with blades at regular intervals, ultrasound, fixed percolated blade, mobile percolated blade, immersion with combined food/solution displacement, single layer drenching with conveyor, recurrent action multilevel drenching, massaging/tumbling, injection of solution into the food and application of solid solute on the food (Ade-Omowaye et al.,2001; Ade-Omowaye et al.,2002).
Process duration
In general, as the time of osmotic treatment increases, the weight loss increases with a decreasing rate (Yang and Le Maguer, 1992;Chaudhary et al., 1993) Different data on osmotic dehydration of different foods show that, water loss, solid gain and weight loss of foods during osmotic dehydration are related to time and come to equilibrium with respect to time. (Azuara et al., 1992; Lazarides et al., 1995)
Process pressure
The pressure gradient in the osmotic dehydration process changes the rates of water loss and solid gain. The hydrodynamic mechanism describes the change in the mass transfer by expansion or compression of internal pore gas of tissue.
The brief review of osmotic dehydration of several fruits and vegetables has been given in Table 1 and 2. The tables show the variables and their ranges taken for osmotic dehydration of fruits and vegetables.