Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Module 11. Lubricants properties, mechanism, class...
Module 12. Polymers type of polymerization, proper...
Lesson 21 Carbohydrates
21.1 Introduction
Food Chemistry is a major aspect of food science. Food chemistry deals with composition and properties of food and chemical changes it undergoes during handling, processing and storage.
Chemically food consists of carbohydrates, proteins, lipids, vitamins, minerals, preservators, colouring and flavouring reagents of food. In this module we will learn more about them in details.
21.2 Carbohydrates
Carbohydrates are defined as polyhydroxy aldehydes or ketones and their derivatives. Their general formula can be represented as Cx(H2O)y. Carbohydrates are formed in plant cells from carbon dioxide and water in a process called as photosynthesis. Light energy is thus trapped in substances which provide energy to living beings. 1 g of carbohydrates provide 4 kcal energy. In human food, the sources of carbohydrates are cereals, roots, tubers, sugarcane and sugarbeet. Carbohydrates are named according to sources from which they are obtained e.g., fructose (fruit sugar), lactose (milk sugar), xylose (wood sugar) and cellulose (from cell membrane).
21.3 General properties of Carbohydrates
21.3.1 Most of the monosaccharides, disaccharides and lower polysaccharides are sweet in taste, whereas starch and cellulose are tasteless.
21.3.2 They exhibit stereoisomerism. Stereoisomers are compounds whose structural formula is same but arrangement of atoms in 3-Dimensional space is different. E.g., with respect to Carbon no. 5, Glucose has two forms, D-glucose and L-glucose.
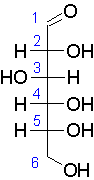 |
|
| D-Glucose |
21.3.3 They exhibit optical activity. Optical activity is a phenomenon in which a molecule rotates plane of plain polarised light. If the molecule rotates the plane towards left, it is designated levo rotatory (- sign) and if the plane is rotated towards right, the molecule is designated dextro rotatory (+ sign). D-glucose rotates plane of plain polarised light towards right while L-fructose rotates it towards left.
21.3.4 The sugars in which free aldehyde or ketone groups are present can reduce Fehling’s solution and Benedict’s solution are called reducing sugars. The sugars in which free aldehyde or ketone groups are absent are called as non-reducing sugars. Glucose, fructose, lactose etc., are reducing sugars whereas sucrose is a non-reducing sugar.
21.4 Functions of Carbohydrates
21.4.1 They are a major part of total caloric intake.
21.4.2 Starch and glycogen serve as temporary stores of glucose.
21.4.3 Water insoluble carbohydrates are major components of cell wall in plant cells and cell membrane in animal cells.
21.4.4 Carbohydrates function as lubricants in skeletal joints and help the cells to stick with each other and remain in place.
21.5 Classification of carbohydrates
Carbohydrates are generally classified into monosaccharides (simple sugars), oligosaccharides (containing few sugar units) and polysaccharides (containing many sugar units).
21.5.1 Monosaccharides
Monosaccharides are sugar molecules containing short chain of carbon atoms, one aldehydic or ketonic group and hydroxyl groups attached to remaining Carbon atoms. If they contain aldehyde group, they are named as aldoses and if they contain ketone group they are named as ketoses. Depending on number of Carbon atoms, monosaccharides are named astrioses (3 C atoms), tetroses (4 C atoms), pentoses (5 C atoms), Hexoses (6 C atoms) and so on.
21.5.1.1 Important Monosaccharides
1. D-glucose or Dextose: It is the building block of starch and cellulose, source of energy for the animals.
2. Fructose: It is found in fruits and honey. Alongwith glucose, it is present in cane sugar (sucrose).
3. Galactose: It is not found in free state in foods but it is component of milk sugar (lactose) and agar-agar.
4. Arabinose and xylose : They are pentoses and found in plant gums.
21.5.1.2 Properties of monosaccharides
1. They are called as reducing sugars because they reduce Cu2+ and Fe(CN)63+ ions.
2. The reducing sugars are oxidized to sugar acids. E.g., Glucose is oxidized to gluconic acid (monocarboxylic acid) and glucaric acid (dicarboxylic acid).
3. Reduction of monosaccharides yield sugar alcohols.
21.5.1.3 Monosaccharide derivatives
These are sugar molecules in which one or more OH groups are replaced by other groups. They can be classified as
Amino sugars: The –OH group is replaced by –NH2 group. E.g. glucosamine, sialic acid.
Deoxy sugars: In deoxy sugars, one –OH group is replaced by –H atom. L-Rhamonse and L-fucose are present in food polysaccharides. 2-deoxy D-ribose is constituent of DNA.
Glycosides: They are condensation products of sugar (glucose) with another molecule. If the second molecule is also a sugar, O-glycoside is formed. If the second molecule has amino group, product is N-glycoside and if the second molecule has thiol group, the product is S-glycoside.
21.5.2 Oligosaccharides
Oligosaccharides are formed by polymerisation of monosaccharide molecules by elimination of water molecules. Depending on number of monosaccharide units, oligosaccharides are classified as di-, tri- tetra-saccharides etc.The linkage between monosaccharide units in oligosaccharide units is glycosidic linkage.
21.5.2.1 Important oligosaccharides
Sucrose: It is most abundant in sugarcane and sugarbeet. It consists of one molecule of glucose and one molecule of fructose, joined together by glycosidic linkage between their anomeric –OH groups. Sucrose is thus non-reducing sugar.
Maltose: It is the intermediate product of starch hydrolysis. It consists of two molecules of glucose units with glycosidic linkage between anomeric C of one molecule and –OH molecule on C-4 of the other. Maltose is reducing sugar.
Lactose: It is found only in mammalian milk. It consists of one molecule of glucose and one molecule of galactose.
21.5.3 Polysaccharides
They are high molecular weight substancescomposed of large number of moosaccharide units combined to form one large polymer molecule. They may be straight chain or branched chain polymers.
21.5.3.1 Important Polysaccharides
Starch: It occurs in cereal grains, pulses, tubers, fruits etc. It is the major source of energy in diet of man. Starch is a polymer of glucose. Starch is a mixture of two substances, about 25% amylose (straight-chain polymer) and 75% amylopectin (branched chain polymer). Because of the structural differences between amylose and amylopectin, they exhibit different properties. Amylose contributes to gel-formation while amylopectin is responsible for thickness properties of foods containing starch.
Dextrin: It is the product of partial hydrolysis of starch. Their properties are intermediate between those of sugars and starch. They are produced when starch is subjected to dry heat. E.g. during toasting of bread.
Glycogen: Glucose is stored in the form of glycogen in animal tissues. It is mainly found in liver and muscles. It is similar in structure to amylopectin but is more branched than amylopectin.
Cellulose: It is a straight chain polymer of glucose molecules. The glycosidic linkages are different in cellulose and amylose. Cellulose is insoluble in water. It is not digested in human tract. Modified cellulose is used in food industries. Carboxymethyl cellulose is used in ice cream.
Pectic substances: They are constituents of plant cell wall. They are mixtures of polysaccharides formed from galactose, arabinose and galacturonic acid. Pectin, a water soluble component is present in fruits and forms gel with sugar and acid.
Gums: They are hydrophilic substances that give a viscous solution or dispersion when treated with hot or cold water. Starches, pectcic substances and derivatives of starch and cellulose are included in this category. They act as thickeners, moisture retainers, emulsion stabilizers, foam stabilizers, clarifying agents, etc. Important gums include Gum arabic (obtained from Acacia plant), Agar (obtained from red-brown algae) and algin (obtained from brown algae).
References & Further Reading:
1. Satyanarayana U. & Chakrapani U. (2011), “Biochemistry”. Books and Allied (P) Ltd. pp. 9-26.
3. N. Shakuntala Manay & M. Shadaksharaswamy (2001), “Foods: Facts and Principles”. New Age International Publishers, pp. 15-32.