Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Module 11. Lubricants properties, mechanism, class...
Module 12. Polymers type of polymerization, proper...
Lesson 22 Lipids
22.1 Introduction
Lipids are defined as substances which are insoluble in water but soluble in solvents of low polarity like chloroform, carbon tetrachloride and ether. This definition of lipids includes many different compounds like terpenes, steroids and fats. They are widely distributed in nature but are not found in free state. They occur in combined state with proteins (lipoprtoeins) or carbohydrates (glycolipids). The greatest amounts of fats present in foods are fats and phospholipids.
22.2 Functions of Lipids
22.2.1 They serve as fuel molecules. 1 gm of fat provides 9 kcal of energy.
22.2.2 Phospholipide and triglycerides are important to structure, composition and permeability of cell wall and cell membrane.
22.2.3 Lipoproteins help in transporting lipids in blood.
22.2.4 Skin waxes act as defense mechanism in avoiding thermal and physical shock.
22.2.5 Fats present in food help to carry fat soluble vitamins so that they are efficiently absorbed.
22.2.6 Fats serve as vitamins, emulsifiers and flavour and aroma compounds.
22.3 Classification of Lipids
Lipids are classified into two groups, simple lipids and compound lipids.
Simple lipids include fatty acids, fats and oils (triglycerides of fatty acids) and waxes (esters of fatty acids with long chain monohydroxy alcohols).
All other lipids like phospholipids, steroids, caretenoids etc. are included in compound lipids.
22.4 Fatty Acids
Fatty acids are long chain carboxylic acids having general formula CH3(CH2)nCOOH. Most of the fatty acids contain even number of Carbon atoms because of the way in which they are synthesized in living beings. But few fatty acids are present which have odd number of Carbon atoms. Most of the fatty acids found in nature are linear, but few branched chain and cyclic fatty acids can also be found.
22.4.1 Saturated Fatty Acids
Straight chain fatty acids containing 2 to 80 Carbon atoms are known, but in food those having 4 to 24 Carbon atoms are present. The largest share is of fatty acids containing 16 & 18 Carbon atoms.
Table 22.1 Saturated fatty acids found in foods
|
No. of C atoms |
Name |
Structure |
|
4 |
Butyric |
CH3(CH2)2COOH |
|
5 |
Valeric |
CH3(CH2)3COOH |
|
6 |
Caproic |
CH3(CH2)4COOH |
|
8 |
Caprylic |
CH3(CH2)6COOH |
|
10 |
Capric |
CH3(CH2)8COOH |
|
12 |
Lauric |
CH3(CH2)10COOH |
|
14 |
Myristic |
CH3(CH2)12COOH |
|
16 |
Palmitic |
CH3(CH2)14COOH |
|
18 |
Stearic |
CH3(CH2)16COOH |
|
20 |
Arachidic |
CH3(CH2)18COOH |
|
22 |
Behenic |
CH3(CH2)20COOH |
|
24 |
Lignoceric |
CH3(CH2)22COOH |
Fatty acids with Carbon atoms 4-10 are present mainly in milk fat, those having 12-24 Carbon atoms are present in vegetable and animal fats & fatty acids having greater than 24 Carbon atoms are found in waxes. In food, lauric, myristic, palmitic and stearic are the most common acids which are present.
22.4.2 Unsaturated fatty acids
Unsaturated fatty acids are fatty acids which contain double bonds between Carbon atoms. They are subdivided into two types MUFA (Mono-unsaturated fatty acid) and PUFA (poly-unsaturated fatty acid). Most of the naturally occuring unsaturated fatty acids are cis-isomers.
Table 22.2 Unsaturated fatty acids found in food
|
Common Name |
IUPAC Name |
Structure |
|
Myristoleic |
9-Tetradecenoic acid |
CH3(CH2)3CH=CH(CH2)7COOH |
|
Palmitoleic |
9-Hexadecenoic acid |
CH3(CH2)5CH=CH(CH2)7COOH |
|
Oleic |
9-Octadecenoic acid |
CH3(CH2)7CH=CH(CH2)7COOH |
|
Linoleic |
9,12-Octadecadienoic acid |
CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH |
|
Linolenic |
9,12,15-Octadecatrienoic acid |
CH3(CH2CH=CH)3(CH2)7COOH |
|
Arachidonic |
5,8,11,14-Eicosatertaneoic acid |
CH3(CH2)3(CH2CH=CH)4(CH2)3COOH |
Oleic Acid is commonly found in all fats. It is present upto a concentration of 75% in Olive oil. Linoelic acid is also commonly found and is present to an extent of 60-80 % in safflowerseed oil. Linolenic acid is found upto an extent of 50-60 % in linseed oil. Arachidonic acid is found in animal sources.
22.4.3 Essential Fatty Acids
Certain fatty acids like lineolic acid are necessary for maintaining healthy conditions of animals. In plants, these acids are formed from oleic acid, but animals lack necessary enzymes for their synthesis. Therefore, they must be supplied from the diet and are known as essential fatty acids.
22.5 Triglycerides:
Triglycerides are carboxylic esters derived from glycerol. Glycerol contains 3 –OH groups which can form ester linkage with fatty acids. If one –OH group is esterified, the compound is called monoglyceride, if two are esterified diglyceride and if all three are esterified, the compound is designated as triglyceride.
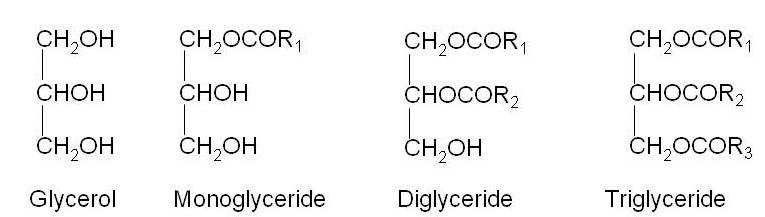
Figure 22.1 Structures of Glycerol, monoglycerides, diglycerides & triglycerides
22.6 Characteristics of lipids
Fats do not have sharp melting points because they are mixture of compounds. As unsaturation of fatty acids increases, the specific heat increases. Viscosity decreases with increasing unsaturation and increases with number of C atoms in fatty acid chain. Solid-liquid ratio can be found out by measuring density of fat. The refractive index increases with increasing unsaturation or with increasing no. of C atoms in fatty acid chain.
22.7 Rancidity
Lipids undergo chemical changes which lead to undesirable odour and flavour. This process is known as rancidity. Two reactions are responsible for rancidity – hydrolysis and oxidation.
Hydrolytic rancidity (lipolytic rancidity) is rancidity developed by hydrolysis reactions in which the lipids are hydrolyzed to yield glycerol and free fatty acids. These reactions can be catalyzed by acids, bases, enzymes or heat. If the reaction is catalyzed by base, salts of fatty acids are obtained and the reaction is known as saponification. Other catalysts release the acids in the free form. Hydrolytic rancidity is important for flavours of milk products, which are due to presence of fatty acids having even number of Carbon atoms, from 4 to 12. Lipolysis depresses the smoke point of cooking oils and as a result, cooking quality of fat is lost, because the foods fried in such oils absorb greater amounts of fat, and the frying quality is also degraded.
Oxidative rancidity is rancidity developed when fats react with oxygen in air, or oxygen producing products. In oxidative rancidity, a large number of intermediates are formed and compounds produced by their condensation are responsible for rancidity. This rancidity is catalyzed by heat, light, ionizing radiatioons and enzymes. This type of rancidity can be prevented by adding substances called anti-oxidants. They function by reacting with intermediates which lead to spoilage of foods.
22.8 Phospholipids
In phospholipids or phosphoglycerides, one of the terminal –OH groups in glycerol is esterified by phosphoric acid and the remaining two by fatty acids. The phosphoric acid is in turn esterified by other compounds like choline, ethanolamine, serine or inositols. Phospholipid containing choline is called lecithin. It is a very good emulsifier and present in egg yolk.
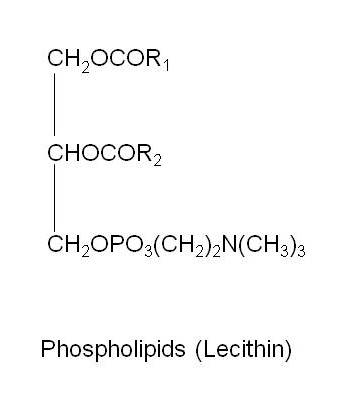
Figure 22.2 Structure of Phospholipid
Phospholipids are components of cell membrane, and influence its structure and function. They help in transporting other lipids in the blood.
References & Further Reading:
1. Satyanarayana U. & Chakrapani U. (2011), “Biochemistry”. Books and Allied (P) Ltd. pp. 28-40.
3. N. Shakuntala Manay & M. Shadaksharaswamy (2001), “Foods: Facts and Principles”. New Age International Publishers, pp. 33-41.