Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Module 11. Lubricants properties, mechanism, class...
Module 12. Polymers type of polymerization, proper...
Lesson 32. Testing methods for polymers
32.1 Introduction
-
Molecular Mass of Polymers
Polymer properties are closely related to their molecular mass, size and structure. The growth of the polymer chain during their synthesis is dependent upon the availability of the monomers in the reaction mixture. Thus, the polymer sample contains chains of varying lengths and hence its molecular mass is always expressed as an average. The molecular mass of polymers can be determined by chemical and physical methods.
- Number Average Molecular Weight
Consider a property which is only sensitive to the number of molecules present, a property that is not influenced by the size of any particle in the mixture. The best example of such properties is the colligative properties of the solutions such as boiling point elevation, freezing point depression and osmotic pressure. For such properties, the most relevant average molecular weight is the total weight of polymer divided by the number of polymer molecules. This average molecular weight follows the conventional definition for the mean value of any statistical quantity. In polymer science, it is called the number average molecular weight - ̅MN.
Then the total weight of all polymers is

and the total number of polymer molecules is

As discussed above, the number average molecular weight is
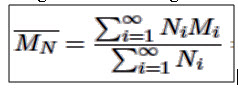
2. Weight Average Molecular Weight
Consider of polymer property which depends not just on the number of polymer molecules but on the size or weight of each polymer molecule. A classic example is light scattering. For such a property we need a weight average molecular weight. To derive the weight average molecular weight, replace the appearance of the number of polymers of molecular weight i or Ni in the number average molecular weight formula with the weight of polymer having molecular weight i or NiMi. The result is

3. Degree of polymerization
The degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule. For a homopolymer, there is only one type of monomeric unit and the number-average degree of polymerization is given by,
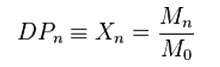
Where, Mn is the number-average molecular weight and M0 is the molecular weight of the monomer unit.
Food Chemistry is a major aspect of food science. Food chemistry deals with composition and properties of food and chemical changes it undergoes during handling, processing and storage.
Chemically food consists of carbohydrates, proteins, lipids, vitamins, minerals, preservators, colouring and flavouring reagents of food. In this module we will learn more about them in details.