Site pages
Current course
Participants
General
Module 1. Phase Rule
Module 2. Fuels
Module 3. Colloids Classification, properties
Module 4. Corrosion Causes, type and methods of p...
Module 5. Water Hardness
Module 6. Scale and sludge formation in boilers, b...
Module 7. Analytical methods like thermo gravimetr...
Module 8. Nuclear radiation, detectors and analyti...
Module 9. Enzymes and their use in manufacturing o...
Module 10. Principles of Food Chemistry
Module 11. Lubricants properties, mechanism, class...
Module 12. Polymers type of polymerization, proper...
Lesson 29. Polymer : Classification and Its use
5.1 INTRODCTION
The word ‘polymer’ is made from two Greek words poly means many and mer means small unit or part. The polymer is defined as very large molecules having high molecular mass. These are also known as macromolecules, which are formed by joining of repeating small structural units on a large scale. The repeating structural units are derived from some simple and reactive molecules known as monomers and are linked to each other by covalent bonds. This process of formation of polymers from respective monomers is called polymerisation. The transformation of ethene to polythene and interaction of hexamethylene diamine and adipic acid leading to the formation of Nylon- 6, 6 are examples of two different types of polymerisation reactions.

29.2 Classification of Polymers :
Polymer can have different chemical structures, physical properties, mechanical behaviour, thermal characteristics etc. and can be classified in different ways.
Natural and synthetic polymer.
Organic and inorganic polymer.
Thermoplastic and thermosetting polymers.
Classification Based on the structure of the polymers.
Addition and condensation polymers.
Homopolymers and copolymers.
Fibres, plastics and elastomers.
Linear branched and cross-linked polymers.
(I) Natural and Synthetic Polymer:
Those polymers which are isolated from natural materials are called natural polymer. e.g. Cotton, silk, wool, and rubber etc.
Those polymers which are synthesized from low molecular weight compounds are called synthetic polymer. e.g. polyethylene, polystyrene, PC, PVA, P.V.C., etc.
(II) Organic and Inorganic Polymer:
A polymer whose backbone chain is made up of carbon atom is known as organic polymer. A polymer whose backbone chain is made up of only inorganic molecules and not of carbon atom is known as inorganic polymers. E.g. Glass, silicone, rubber etc.
(III) Thermoplastic and Thermosetting Polymer:
Thermoplastic: Those polymers which soften on heating and moulded or extruded in to required shapes are known as plastics for the manufacturing of a wide range of articles. E.g. Polyethylene, polypropelene, P.V.C, polystyrene, etc.
The process of heating, reshaping and retaining the same on cooling can be repeated several times.
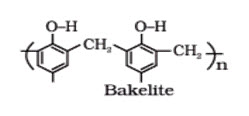
Thermosetting Polymers: A sample of such material is gigantic molecule and heating does not soften it, since softening would require breaking of covalent bond. Indeed, heating may causes formation of additional cross-links and make the material harder, for this reason space network polymer are called thermosetting polymer. Such polymers, upon heating sets into an infusible mass and once sets cannot be reshaped. E.g. Phenol-formaldehyde (Bakelite), Urea-formaldehyde (polyurethane) etc.
(IV) Classification Based on the structure of the polymers
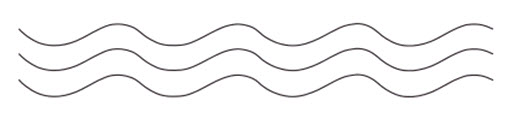
1. Linear polymers
These polymers consist of long and straight chains. The examples of Polymers are high density polythene, polyvinyl chloride, etc. These are represented as:
2. Branched chain polymers
These polymers contain linear chains having some branches, e.g., low density polythene. These are depicted as follows:
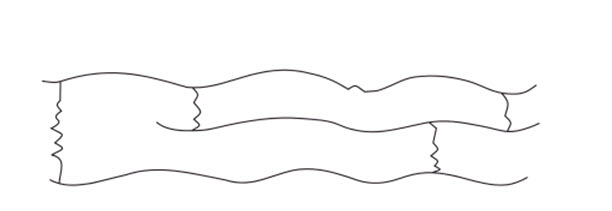
3. Cross linked or Network polymers
These are usually formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains, e.g. phenol-formaldehyde, melamine, etc. These polymers are depicted as follows:

(V) Addition and Condensation polymerization:
Addition polymerization: It is also known as chain reaction polymerization. There is series of reactions each of which consumes a reactive particle and produce another similar particle, each individual reaction thus depends upon the previous one. The reactive particles can be free radicals; cation or anion
CH2=CH2 + Rad. ___________ Rad. –CH2-CH2.
Rad –CH2-CH2. + n( CH2=CH2) __________ [_CH2 _CH2_]- n
Polyethylene
Polymers are formed in two general ways.
(A) Addition polymerization (Chain reaction polymerization)
(B) Condensation polymerization (Step reaction polymerization)
(A) Addition Polymerization: There is series of reactions each of which consumes a reactive particle and produce another similar particle, each individual reaction thus depends upon the previous one. The reactive particles can be free radicals; cation or anion
CH2=CH2 + Rad. ___________ Rad –CH2-CH2.
Rad –CH2-CH2. + n( CH2=CH2) __________ [___CH2 ___CH2 __]- n
Polyethylene
(B) Condensation Polymerization: There is a series of reactions each of which is essentially independent of the preceding one. Formation of polymer takes place because the monomer undergoes reaction at more than one functional group. E.g. A diol reacts with a dicarboxylic acid to form an ester but each moiety of the simple ester still contain a group that can react to generate another ester linkage and hence a large molecule and so on.
(VI) Homopolymers and copolymers
(VII) Classification Based on Molecular Forces
A large number of polymer applications in different fields depend on their unique mechanical properties like tensile strength, elasticity, toughness, etc. These mechanical properties are governed by intermolecular forces, e.g., van der Waals forces and hydrogen bonds, present in the polymer. These forces also bind the polymer chains. Under this category, the polymers are classified into the following four sub groups on the basis of magnitude of intermolecular forces present in them.
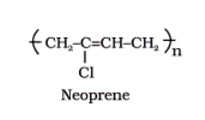
1. Elastomers
These are rubber – like solids with elastic properties. In these elastomeric polymers, the polymer chains are held together by the weakest intermolecular forces. These weak binding forces permit the polymer to be stretched. A few ‘crosslinks’ are introduced in between the chains, which help the polymer to retract to its original position after the force is released as in vulcanised rubber. The examples are buna-S, buna-N, neoprene, etc.
2. Fibres
Fibres are the thread forming solids which possess high tensile strength and high modulus. These characteristics can be attributed to the strong intermolecular forces like hydrogen bonding. These strong forces also lead to close packing of chains and thus impart crystalline nature. The examples are polyamides (nylon 6, 6), polyesters (terylene), polyacrylonitrile etc.

3. Thermoplastic polymers
These are the linear or slightly branched long chain molecules capable of repeatedly softening on heating and hardening on cooling. These polymers possess intermolecular forces of attraction intermediate between elastomers and fibres. Some common thermoplastics are polythene, polystyrene, polyvinyls, etc.
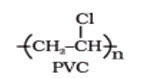
4 Thermosetting polymers
These polymers are cross linked or heavily branched molecules, which on heating undergo extensive cross linking in moulds and again become infusible. These cannot be reused. Some common examples are bakelite, urea-formaldelyde resins, etc.
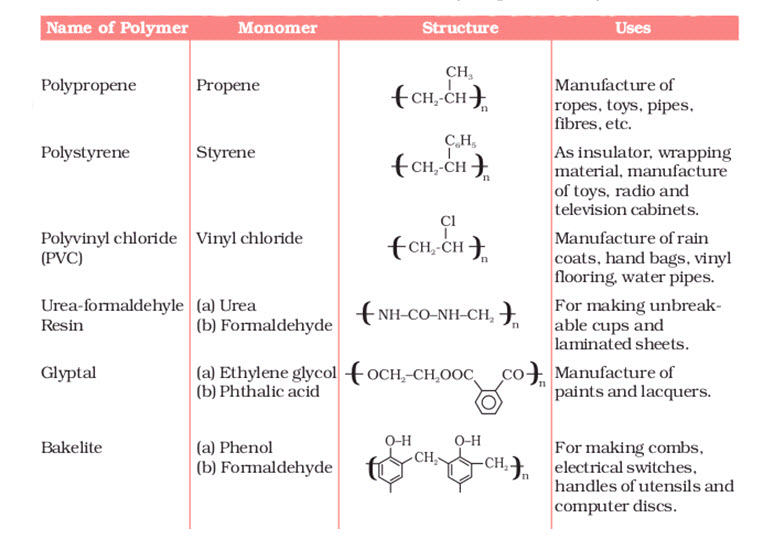
- Polymers of Commercial Importance
Besides, the polymers already discussed, some other commercially important polymers along with their structures and uses are given below in Table 15.1.
The common use of polymers in the manufacture of plastic buckets, cups and saucers, children’s toys, packaging bags, synthetic clothing materials, automobile tyres, gears, electrical components, contact lenses, adhesives, medical supplies (i.e. bone cement and blood begs) and seals, electrical insulating materials and machine parts has completely revolutionised the daily life as well as the industrial scenario. Indeed, the polymers are the backbone of four major industries viz. plastics, elastomers, fibres and paints and varnishes.
(A) Chain Reaction Polymerization:
It is a series of reactions. In which an initiator such as peroxide is added to monomer to form a bigger free radical. This bigger radical adds to another monomer (alkene) to generate a still larger radical and so on. And finally at the chain termination large molecule known as polymer is produced. E.g. Polymerization of vinyl compound it proceeds via three steps.
(i) Chain initiating step: Free radical generated from initiator, adds to the double bond of monomer to form a new bigger free radical.
(a) Peroxide __UV light______ Rad .
(b) Rad . + CH2=CH ___________ Rad-CH2-CH .
| |
G G
(ii) Chain propagation:
(c) Rad-CH2-CH . + CH2=CH _______________ Rad –CH2-CH-CH2-CH .
| | | |
G G G G
(d) Rad–CH2-CH-CH2-CH. +nCH2=CH__ Rad –CH2-CH-[CH2-CH]n__CH2-CH .
| | | | | |
G G G G G G
In the propagation step, radical combines with second monomer molecule. This forms a new radical and then after many similar repetition produce large radical.Thus in each step consumption of free radical is accompanied by the formation of new; bigger free radical.
(iii) Chain Termination:
Reaction chain is terminated by combination or disproportionation of two free radicals.
(a) Combination the two radical may pair their odd electrons to produce a polymer.
Rad _ CH2 _ CH _[_ CH2 _ CH_]_ n CH2 _ CH . + . CH _ CH2 _[_ CH _ CH2_]_ n CH _ CH2 _ Rad
| | | | | |
G G G G G G
Rad _ CH2 _ CH _[_ CH2 _ CH_]_ n CH2 _ CH . __ . CH _ CH2 _[_ CH _ CH2_]_ n CH _ CH2 _ Rad
| | | | | |
G G G G G G
(b) The active site of one radical may strike on the second carbon in the chain of other radical. Here first one radical abstracts an H . from the other radical, which forms a
π-bond and produce a mixture of two polymers.
Rad _ CH2 _ CH _[_ CH2 _ CH_]_ n CH2 _ CH . + . CH _ CH2 _[_ CH _ CH2_]_ n CH _ CH2 _ Rad
| | | | | |
G G G G G G
↓
Rad _ CH2 _ CH _[_ CH2 _ CH_]_ n CH2 = CH + CH2 _ CH2 _[_ CH _ CH2_]_ n CH _ CH2 _ Rad
| | | | | |
G G G G G G
Mechanism of Free radical Vinyl Polymerization.
The polymerization of ethylene and substituted ethylene (i.e. styrene, acrylonitrite, vinyl chloride.)are carried out using free radicals; Which are generated in the presence of a small amount of an initiator such as peroxide. Here the reaction occurs at the doubly bonded carbons i.e. at the vinyl groups and hence is called vinyl polymerization. E.g.
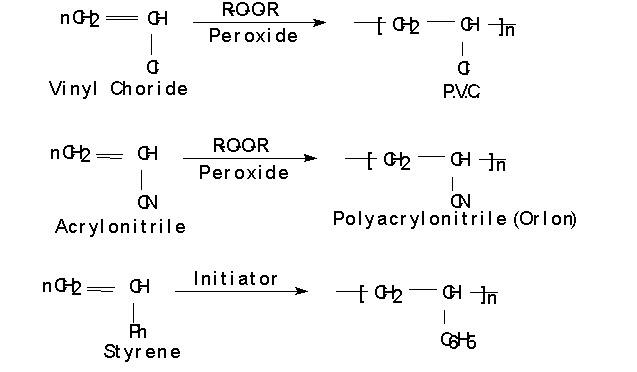
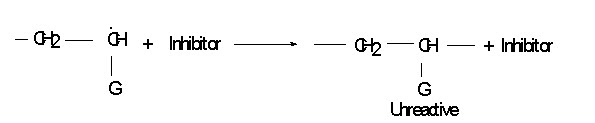
Inhibitor:
When compound such as phenols, quinones and some amines are added during polymerization process the growing free radical may react with such compound and therefore reaction chain is terminated, but no new one begun. Such compound is known as inhibitor.
Synthesis of NYLON-- 6,6
It is an example of step reaction Polymerization. In which Carboxylic acid that contains more than one __COOH group reacts with an amine that contains more than one __NH2 group then the products are polyamides. Nylons are aliphatic polyamides. There are different types of nylons and are indicated by numbering systems. This numbers gives the number of carbon atom present in (first) the diamines and (second) in the dicarboxylic acid.
NYLON--6,6: It is prepared by the condensation between adipic acid and 1,6-hexanediamine.
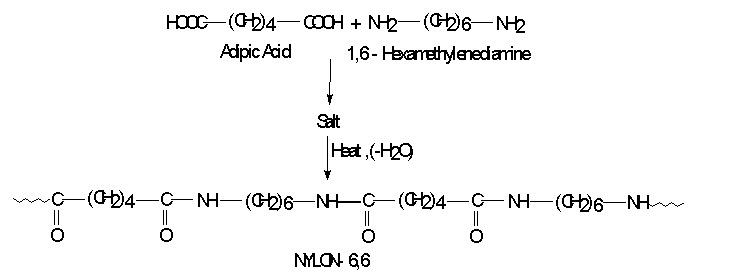
It is used as plastic as well as fibres. As a plastic it is used in gears and bearings. As a fiber it is used in tyres cord and rops.
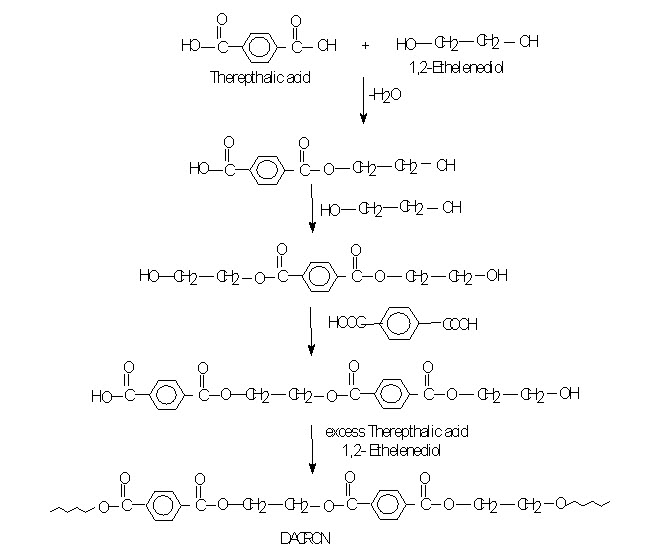
POLYETHYLENE TEREPHTHALATE (PETP) (Terylene, Dacron): When Carboxylic acid or it’s ester that contains more than one __COOH group or __COOR group reacts with diol then the products are,
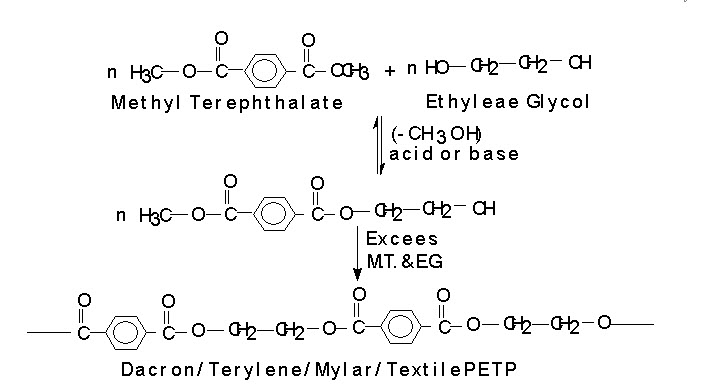
They are use as fiber. It is commonly known as terylene. PETP is also used in to films of magnetic recording tapes. The material in sheet form also marketed as Malar.
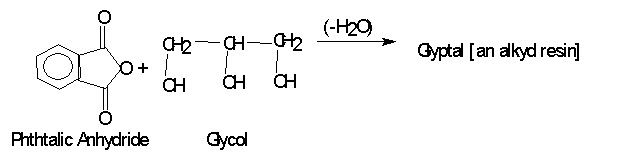
If each monomer molecules contains just two functional groups, growth can occur in only two direction and a linear polymer is obtained as in Dacron. But if reaction can occur at more than two positions in a monomer, there is a formation of a highly cross linked space network polymer as in glyptal, an alkyd resin.
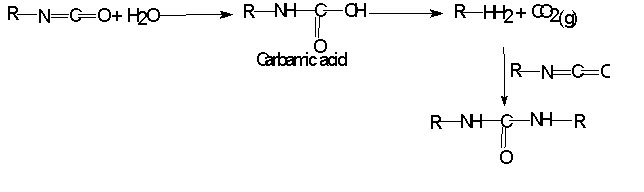
Step Reaction Polymerization
Step reaction polymerization proceeds through a series of reaction in which two monomers each of which contains two functional groups are combined to produced high molecular weight substance and a small molecule like H2O, HCl or CH3OH.
Phenol - Formaldehyde (Bakelite) resins are formed by the poly condensation between phenol and formaldehyde. It can be catalysed by either acids or base.
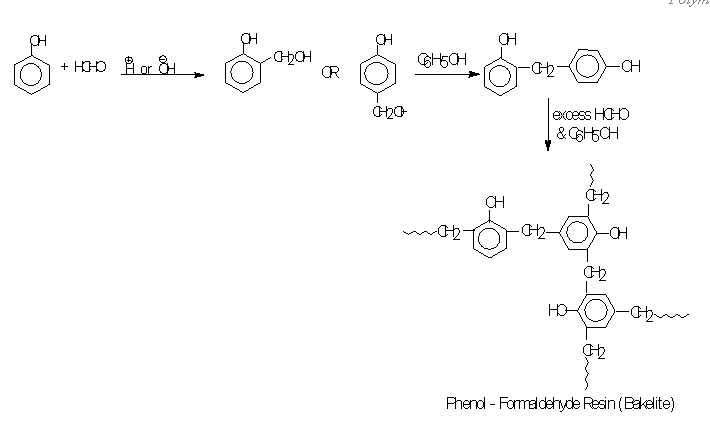
when phenol is treated with formaldehyde in presence of alkali or acid, there is obtained a high molecular weight Phenol – formaldehyde resin, in which many phenol resin are held together by -CH2 groups. The stages involve in the formation of polymer seem to be the following. First phenol reacts with formaldehyde to form O- or P- hydroxylmethylphenol. Hydroxymethylphenol then reacts with another molecule of phenol, with the loss of H2O, to form a compound in which two rings are joined by a -CH2 link. This process is then continuous to yield a product of high molecular weight. Since three position in each phenol molecule are susceptible to attack, the final product contains many cross links and hence has a rigid three-dimensional structure. It is thus a space network polymer.
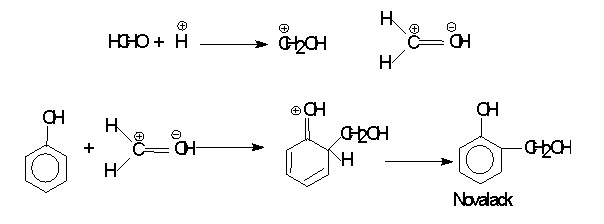
Mechanism of Phenol Formaldehyde
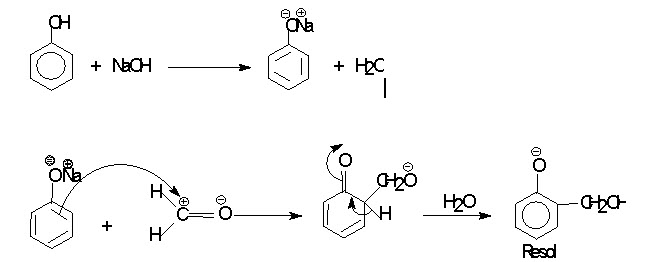
Phenol-Formaldehyde (PF) resins are formed by the polycondensation between phenol and formaldehyde. It is catalysed by either acid or base. It proceeds via electrophilic aromatic substitution. The first stage can be viewed as both electrophilic substitution on the ring by the electron deficient carbon of formaldehyde, and nucleophilic addition of the aromatic ring to the carbonyl group. Acid catalyzes reaction by protonating formaldehyde and increasing the electron deficiency of the carbonyl carbon. While base catalyzes reaction converting phenol in to more reactive phenoxide ion.
MECHANISM: (a) The acid catalysed reaction:
(b) Base catalysed reaction :
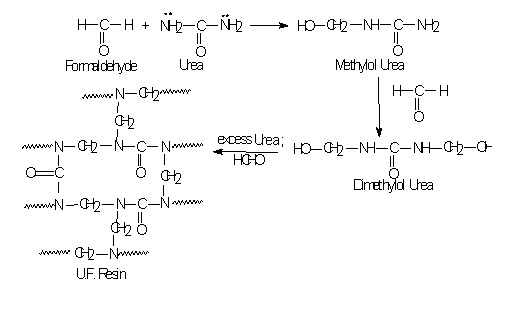
Urea – Formaldehyde Polymer:
When urea reacts with formaldehyde it produces methylol urea, dimethylol urea and so on. It is also step reaction polymerization. When excess urea and formaldehyde are their it will results in to three dimensional space - network Urea formaldehyde polymer. It is highly important molded plastic. Also used in textile finishing.
Polyurethane
When dihydroxy alcohols reacts with diisocyanaates will result in polyurethane are;
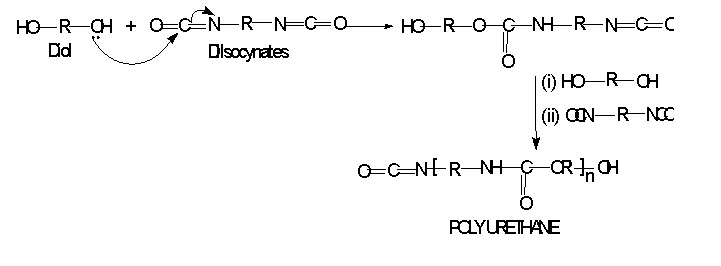
It is linear polymer and have a wide industrial application upon hydrolysis it gives polyurethane form.
CO-POLYMERIZATION
When a mixture of two or more different monomer is allowed to undergo polymerization, will results in to co-polymer. OR A polymer that contains two or more different kind of monomeric units in the same molecule are known as co-polymer. If the polymer is prepared from different type of monomers (M1 & M2) then the process is known as CO-POLYMERIZATION.
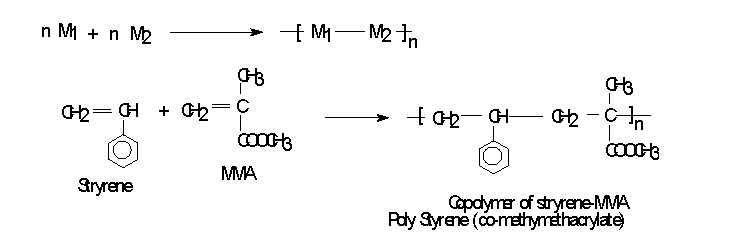
By the process of copolymerization the polymer can be made with different properties compare to homopolymerization. E.g. Polystyrene alone have a good electric insulation properties and can be molded in to parts for radios, television sets and automobiles etc. But when styrene is copolymerized with 30% butadiene adds toughness to the polymer. Styrene with acrylonitrite with (20-30%) increase resistance to impact and to hydrocarbons(solvent). Styrene with maleic anhydride produce polymer which on hydrolysis becomes water soluble and is used as a dispersant and sizing agent. Styrene with 75% butadiene produce an elastomer .
CLASSIFICATION OF COPOLYMER IN DETAIL.
If two monomers M1 & M2 are polymerized together four arrangements are possible in the polymer structure.
(i) Alternating copolymer.
(ii) Random copolymer
(iii) Block copolymer
(iv) Graft copolymer
(i) Alternating copolymers : When the two monomer units alternatively in a linear fassion the product so obtained is called alternating copolymers.
![]()
e.g. Styrene and methylmethacrylate.
(ii) Random copolymer : When the two monomer are distributed randomly among the chain the product so obtain is called random copolymer.
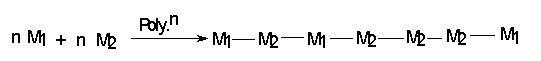
e.g. Styrene and butadiene.
(iii) Blocke copolymer : When the two monomer get inited in a block made up of one monomer alternate with block of another is called block copolymer. Here section made up of one monomer alternate with section of another.
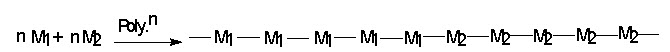
e.g. Ethylene oxide & Ethelene Terphthalate
(iv) Graft copolymer : A branch of one kind is grafted to a chain of another kind. The one monomer unit can be grafted (i.e. added in the form of branched chain) on the linear chain of a polymer molecules. Such branched polymer are known as graft copolymer
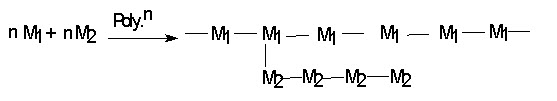
E.g. Natural rubber and acrylonitrite.
Plastic: The molecular structure of plastics is of two general kinds. The linear and branched polymers may be more or less crystalline, and include some of the materials also used as fibers: nylon, e.g. They include the various polyalkenes we have mentioned: polyethylene, poly(vinyl chloride), polystyrene, etc. on heating, these polymers soften, and for this reason are called Theromoplastic. It is in this softened state that they can be molded or extruded.
Space-network polymers (or resins) are highly cross-linked to form a rigid but irregular three-dimensional structure, as in phenol-formaldehyde or urea-formaldehyde resins. A sample of such material is essentially one gigantic molecule; heating does not soften it, since softening would require breaking of covalent bonds. Indeed, heating may cause formation of additional cross-links and thus make the material harder; for this reason, these polymers are called Thermosetting polymers. This continuation of the polymerization process through heating is often coupled with the shaping of the product.
Some Important Definitions :
(a) Monomer : The individual small molecules from which the polymer is formed are known as monomers.
(b) Polymer : Polymers are macro molecules (giant) having high molecular weight and are made up of a great many simpler units – identical to each other or at least chemically similar – joined together in a regular way.
(c) Polymerization : The process by which the monomer molecules are linked together to form a bigger molecule (macro-) is called polymerization.
(d) Degree of polymerization : The number of repeating unit present in the polymer chain is called degree of polymerization (n).
(e) Plastics : When a polymer is shaped in to a hard and though utility articles by the applications of heat and pressure then it is used as plastics. E.g. P.V.C. polymethyl methacrylate (P.M.M.A.), poly styrene etc. which can be extruded as a sheets or pipes.
(f) Fibers : Fibers are long, thin, thread like bits of material that are having great tensile (pulling) strength in the direction of the fiber. E.g. nylon, terylene, polyester etc.
(g) Elastomers : Elastomer possesses the high degree of elasticity that is characteristic of rubber. It can be greatly deformed and stretched up to eight times of its original length and yet return to its original shape. E.g. natural rubber, synthetic rubber, silicon rubber.
(h) Co-polymer : Polymers which are made up of two or more different kind of monomers are known as copolymer e.g. styrene- butadiene copolymer.
(i) High polymers: Polymer which contains more than hundred monomers in their backbone is called high polymers.
(j) Initiator : Initiator is species which are responsible to initiate chain reaction by breaking down itself to form a free radical in presence of U.V. light or heating.