Site pages
Current course
Participants
General
MODULE 1. Overview of renewable energy sources
MODULE 2. Characterization of Biomass
MODULE 3. Thermochemical conversion Technology (TCCT)
MODULE 4. Biochemical conversion Technology-Biogas...
MODULE 5. Bio-fuels (BCCT)
MODULE 6. Solar Energy Conversion System (SECS)
MODULE 7. Hydro-Energy Conversion System (HECS)
MODULE 8. Wind Energy Conversion System (WECS)
MODULE 9. Ocean Energy Conversion System (OECS)
MODULE 10. Energy conservation in agriculture
LESSON 8. Biomass Gasification Methods
Thermochemical conversion method – Principle –Gasifiers – Operation-types-Applications
The thermo-chemical decomposition of hydrocarbons from biomass in a reducing (oxygen deficient) atmosphere is called gasification. The resulting gas product contains combustible gases – hydrogen (H2) and carbon monoxide (CO) as the main constituents. Byproducts are liquids and tars, charcoal and mineral matter (ash or slag). Reducing atmosphere of the gasification stage means that only 20% to 40% of stochiometric amount of oxygen (O2) related to a complete combustion enters the reaction. This is enough to cover the heat energy necessary for a complete gasification. It creates sensible heat necessary to complete gasification from its own internal resources.
Biomass gasification is basically the conversion of solid biomass such as wood, agricultural residues etc., into a combustible gas mixture normally called “producer gas” (or Low Btu gas). The solid biomass is partially burnt in the presence of air or oxygen to produce a low or medium calorific value gas. Partial combustion process occurs when air supply is less than adequate for combustion of biomass to be completed. Given that biomass contains carbon, hydrogen and oxygen molecules, complete combustion would produce carbon dioxide and water vapour. Partial combustion produces carbon monoxide as well as hydrogen which are both combustible gases
Solid biomass fuels are usually inconvenient, have low efficiency of utilisation and can only be used for certain limited applications. Combustion is the normal conversion process used in cooking, heating space and water, or generation of steam usually with low efficiencies, generation of power, for example, requires high/medium pressure steam boiler along with steam engine or turbine with accessories. For small power needs (a few kilowatts to few hundred kilowatts), this conversion technology is not only capital intensive and complex, but also very inefficient.
Conversion of the same biomass to a combustible gas mixture called producer gas removes most of these problems associated with the use of solid biomass fuels. While conversion to gas results in loss of energy of upto 25 percent, use of gas can be highly efficient and hence overall efficiency could be very high. Also it can be employed at any scale and hence is ideally suited for decentralised application whether for shaftpower, electricity or thermal energy.
Biomass Gasifier-an attractive technology option
Renewed emphasis on increased use of bio-resources does not obviously imply going back to traditional, inefficient and inconvenient techniques and devices. These resources obviously need to be utilised in keeping with modern-day conveniences and efficiencies that people are so used to by now. This indeed is the most basic and vital difference between the past and expected future scenario in which biomass is seen as playing an important role.
Numbers of thermal applications of gasifier systems have shown adequate and immediate promise. These applications involve diverse situations: situation where biomass might already be in use with traditional technologies; situations where biomass may not be currently in use but is available as a by-product; and situations where biomass may need to be procured for a switch over from fossil fuel. However even in situations where thermal energy is currently being provided by bio-resources, careful study of the application and effective development of application packages becomes necessary
The gas from the gasifier burns completely; it is clean, odourless and colourless. The fuel gas can be directly mixed with cold air to generate hot air at desired temperature for purpose of drying. Furthermore the producer gas can also be used to replace diesel (upto 70-75 percent) in standard diesel engine for shaft power generation comparable to conventional electricity at substantially lower cost than for diesel based generation. The gasifier is also environment friendly equipment without any harmful emission.
Biomass as a Source of Energy
The world over, biomass fuels represent the second largest source of energy used after fossil fuels. In developing countries about two thousand million people rely almost entirely on biomass fuels for their energy needs. It represents about 35 percent of the energy used. About 40 percent of the total energy consumed in India, even today, comes from fuelwood, charcoal and various agricultural residues. About half of all the trees cut in the world , for whatever reasons, end up being used as fuel for cooking and heating. The present methods for utilization of these resources are highly inefficient. On the other hand, utilization of the residues through gasification route becomes economical and promising for thermal and power to rural areas and for small scale agro industries. This will also reduce the pressure on the worsening fuel wood situation. Agro residues are available abundantly and can also be used for gasification.
Prevailing chemical reactions are listed in Table 1, wherein the following main three gasification stages are described.
Stage I. Gasification process starts as auto-thermal heating of the reaction mixture. The necessary heat for this process is covered by the initial oxidation exothermic reactions by combustion of a part of the fuel.
Stage II. In the second - pyrolysis stage, being passed through a bed of fuel at high temperature pyrolyzes combustion gases. Heavier biomass molecules distillate into medium weight organic molecules and C02. In this stage, tar and char are also produced.
Stage III. Initial products of combustion carbon dioxide (CO2) and (H2O) are reconverted by reduction reaction to carbon monoxide (CO), hydrogen (H2) and methane (CH4). These are the main combustible components of producer gas. These reactions, not necessarily related to reduction, occur at high temperature. Gasification reactions, most important for the final quality (heating value) of syngas, take place in the reduction zone of the gasifier. Heat consumption prevails in this stage and the gas temperature will therefore decrease. Tar is mainly gasified, while char, depending upon the technology used, can be significantly "burned", reducing the concentration of particulates in the product.
|
Gasification Stage |
Reaction formula |
Reaction heat |
|
Stage 1: |
C+1/2O2® CO |
Exothermal |
|
Oxidation and Other exothermic Reaction |
CO + 1/2O2® CO2 C + O2 ® CO2 C6H10O5 ® xCO2 + yH2O H2 + 1/2O2 ® H2O CO + H2O ® CO2 + H2 CO + 3H2 ® CH4 + H2O |
|
|
Stage II : Pyrolysis |
C6H10O5 ® CxHz + CO C6H10O5 ® CnHmOy |
Endothermic |
|
Stage III : Gasification (Reduction) |
C + H2O ® CO + H2 C + CO2 ® 2CO CO2 + H2 ® CO + H2O C + 2H2 ® CH4 |
Endothermic
Exothermic |
Table 1: Biomass gasification chemical reactions
(Source : J.B. Jones & C.A. Hawkins. Engineering Thermodynamics, 1986,P. 456)
The net product of air gasification can be found by summing of the partial reactions, as follows:
Carbohydrate matter
(C6H10O5) + O2 ® CXHY + CLHMON +CO +H2 +Heat
Reactions labeled in Table 2 as exothermic means that chemical energy is converted to sensible heat and reactions labeled as endothermic means that heat is consumed in favor of chemical energy.
Design of gasifier depends upon type of fuel used and whether gasifier is portable or stationary. Gasifiers are classified according to how the air blast introduced in the fuel column. History of gasification reveals several designs of gasifiers. The fixed bed gasifier has been the traditional process used for gasification, operated at temperatures around 1000 C. The most commonly built gasifiers are classified as:
Updraft gasifiers (fig 1)
Downdraft gasifiers (fig 2)
Crossdraft gasifiers (fig 3)
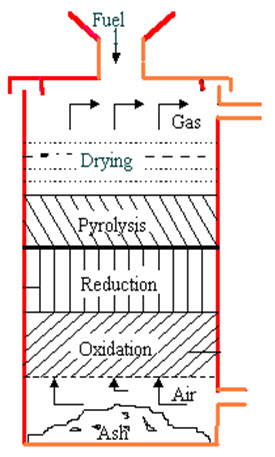
Fig.1. Updraft Gasifier
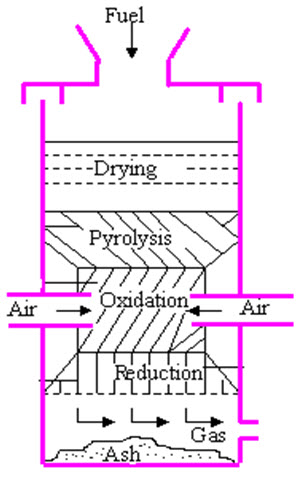
Fig.2 Downdraft Gasifier
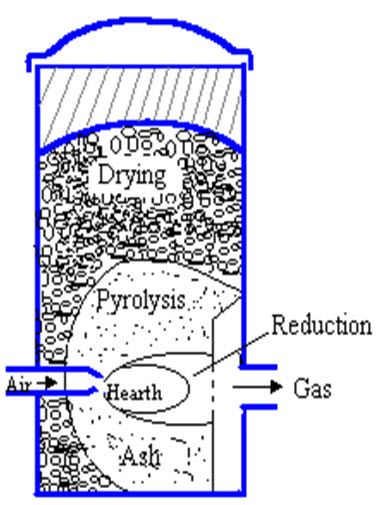
Fig.3. Crossdraft gasifier
Fluidized bed Gasification
Fluidized bed gasification has been used extensively for coal gasification for many years, its advantage over fixed bed gasifiers being the uniform temperature distribution achieved in the gasification zone. The uniformity of temperature is achieved using a bed of fine grained material into which air is introduced, fluidizing the bed material and ensuring intimate mixing of the hot bed material, the hot combustion gas and the biomass feed.
Two main types of FB gasifier are in use;
1.Circulating fluidized bed,
2.Bubbling bed.
A third type of FB is currently being developed, termed a fast, internally circulating gasifier, which combines the design features of the other two types. The reactor is still at pilot stage of development.
Circulating Fluidized bed Gasification
Circulating FB gasifier are able to cope with high capacity throughputs and are used in the paper industry for the gasification of bark and other forestry residues. The bed material is circulated between the reaction vessel and a cyclone separator, where the ash is removed and the bed material and char returned to the reaction vessel. Gasifiers can be operated at elevated pressures, the advantage being for those end use applications where the gas is required to be compressed afterwards, as in a gas turbine.
Bubbling bed gasification
Bubbling bed FB gasifiers consist of a vessel with a grate at the bottom through which air is introduced. Above the grate is the moving bed of fine-grained material into which the prepared biomass feed is introduced. Regulation of the bed temperature to 700-900oC is maintained by controlling the air/biomass ratio. The biomass is pyrolysed in the hot bed to form a char with gaseous compounds, the high molecular weight compounds being cracked by contact with the hot bed material, giving a product gas with a low tar content, typically < 1-3 g/Nm3.