Site pages
Current course
Participants
General
Module 1: Basics of Agricultural Drainage
Module 2: Surface and Subsurface Drainage Systems
Module 3: Subsurface Flow to Drains and Drainage E...
Module 4: Construction of Pipe Drainage Systems
Module 5: Drainage for Salt Control
Module 6: Economics of Drainage
Keywords
Lesson 11 Drainage of Irrigated, Humid and Coastal Regions
11.1 Introduction
Besides forming the mineral constituents of various size ranges, the weathering and the chemical composition of the parent rock decide the chemical characteristics (neutral, acidic or alkaline) of a soil mass. These fundamental soil chemical properties undergo major changes, particularly at the surface and in shallower depths during various soil treatments through human intervention and the influence of climatic factors such as evaporation and high rainfall. Thus, a neutral soil may become saline or alkaline over a time period. On the other hand, rainfall infiltration during the monsoon washes out and also leaches down the salts, causing a temporary reduction in salt concentration on the land surface and at shallow depths. Organic matter and its decomposition, oxidation of sulphur compounds in the soil, washing out of cations present in the soil in high rainfall regions and irrigation using acidic water may turn the soil acidic (Bhattacharya and Michael, 2003).
The chemical behavior of the soil and the various reactions that may take place between soil, various chemicals present in the soil water and the chemicals that may be added to it from outside the soil system, are governed by the surface properties of the soil particles. The larger is the surface area per unit mass of the soil, the more chemically active the soil will be (Bhattacharya and Michael, 2003). The total surface area of clay per unit mass is much greater than that of sand, and hence clay is highly active chemically. Such chemical activity and the resulting chemical changes in the clay component of the soil have profound influence on the physical behavior of the soil. However, the chemical activity involving sand and silt are practically of no major consequence on the physical behavior of the soil (Bhattacharya and Michael, 2003).
Soil is the natural base for all biological activities, including agriculture. Many of the chemical problems of soils are found in the irrigated areas of the world (including India). Maintaining a favorable chemical regime in the soil is very important for a successful and sustainable agriculture. Broad groups of Indian soils and their brief description are presented in Table 11.1.
Table 11.1. Major soil groups in India (Bhattacharya and Michael, 2003)
|
Sl. No. |
Soil Type |
Area (Mha) |
Soil Taxonomic Group |
Major Places of Occurrence |
Properties and Suitability |
|
1 |
Alluvium Soils |
50 |
Entisol, Alfisol, Inceptisol, Aridisol. |
Punjab, Haryana, Uttar Pradesh, Madhya Pradesh. Bihar, West Bengal, Assam, Coastal regions. |
Coarse to fine, permeable, can support most agricultural crops, slightly alkaline. In Assam, it is slightly acidic due to high rainfall. |
|
2 |
Black Soils |
54 |
Vertisol, Entisol. |
Maharastra, Madhya Pradesh, Gujarat, Rajasthan, Andhra Pradesh, Tamil Nadu. |
Fine texture, slow in water transmission, cracks when dry, poor workability, supports cotton, sorghum, millet, wheat, soybean, sugarcane. |
|
3 |
Desert Soils |
29 |
Calciorthids, Lithic Entisols, Psamments |
Rajasthan, Haryana, Punjab. |
Fine to medium texture, alkaline, saline, can support crop with irrigation. |
|
4 |
Forest and Hill Soils |
55 |
Aridisol and Entisol |
Sikkim, Himachal Pradesh, Kashmir. |
Coarse to fine, permeable, slightly acidic, supports maize and wheat. |
|
5 |
Laterites and Lateritic Soils |
24.8 |
Oxisol, Ultisol, Alfisol |
Orissa, Maharastra, Tripura, West Bengal, Karnataka, Andhra Pradesh, Bihar. |
Coarse to medium texture, varying water transmission rate, slightly acidic, hard when dry, supports tea, cardamom, rubber, paddy, arecanut. |
|
6 |
Peat and Marshy Soils |
0.015 |
Histosol |
Kerala |
High water holding capacity, acidic, supports rice, coconut. |
|
7 |
Red Soils |
55 |
Alfisol, Ultisol |
Tamil Nadu, Karnataka, Maharastra, Andhra Pradesh, Goa, Pondichery, Bihar, West Bengal, Assam, Uttar Pradesh, Rajasthan. |
Generally loam to clay loam, slightly acidic to slightly alkaline, good water transmission property, supports a large variety of field crops and cash crops. |
11.2 Problematic Soils and Salient Terminologies
11.2.1 Overview of Problematic Soils
All soils contain some amount of salts. Salinization of soil refers to further increase in the natural salt concentration in the soil. Soil salinization has been identified as a major process of land degradation (FAO, 2000). The greatest technical causes of decreasing production in many irrigation command areas, particularly in the arid and semi-arid regions, or failure of large rainfed agricultural areas are waterlogging, salinization, and development of alkali lands. Management of salt-affected soils requires a combination of engineering and agronomic measures based on a careful analysis of the causes of salt problem, behaviour of different crop plants under saline environment, the current and desired production level from the land, the alternate technologies available to tackle the salt problem and the costs and benefits expected in the adoption of a certain technology package (Bhattacharya and Michael, 2003). Note that all types of salt-affected soils may not be subjected to reclamation, which may prove to be quite expensive.
From the agricultural viewpoint, a soil is called salt affected when its salt concentration starts adversely affecting seed germination, plant growth, and crop yield. The extent of adverse effects varies with the type of soil, plant species, stage of plant growth, and the type of salt present in the soil. There is a large inter-seasonal variability of the concentration of salt in the soil profile and groundwater due to temporal variation in rainfall and evaporation. Despite all these variations, it is desirable to classify the soils chemically. The salt-affected soils can be grouped into three classes: (a) saline soil, (b) alkali or sodic soil, and (c) saline-alkali soil. Acid soils belong to a different class and may or may not be saline.
In India, the salt-affected soils are found in the irrigated semi-arid regions, arid and semi-arid western part of the country and in several places in southern India (Bhattacharya and Michael, 2003). In addition, the soils in the large coastal belt suffer from coastal salinity. Acidic soils are found in the high rainfall zones of north-eastern India. Highly acidic soils are found in the south-western state of Kerala. The details about the classification of salt-affected soils and their occurrence in India are given in Lesson 14.
11.2.2 Salient Terminologies
The terminologies characterizing the chemical properties of soil and water are briefly described in this section.
(1) Electrical Conductivity (EC)
Soil water (i.e., water present in soil pores) is a solution of different salts, mainly Chlorides, Sulphates, Carbonates, and the Bicarbonates of Sodium, Potassium, Calcium and Magnesium. Therefore, the soil water is a conductor of electricity. There may be many other salts also in solution in the soil water, but their proportions are much smaller than the above-mentioned constituents. Since the salt concentration in the soil water has a direct bearing on the performance of plant, electrical conductivity (EC) of the soil solution, usually denoted as ECe (EC of soil saturation extract), is a widely used soil chemical parameter to describe the salinity status of the soil and its influence on plant growth.
The usually adopted units for expressing electrical conductivity are mmhos/cm (millimhos per centimetre), mmho/cm (micromhos per centimetre) and dS/m (deciSiemens per metre); the last one is most widely used these days. Numerically, 1 mmhos/cm = 1 deciSimens per metre. The salt concentration of the soil solution is also expressed as total dissolved solids (TDS) in units such as in parts per million (ppm), milligram per litre (mg/L), milliequivalent per litre (meq/L or me/L), or in parts per hundred (percent). The average and approximate interrelation (adequate for drainage studies) between EC and TDS is as follows (Bhattacharya and Michael, 2003): EC of 1 dS/m = 640 mg/L of TDS = 640 ppm = 10 meq/L. Also, note that EC of 1 dS/m = 1 mmhos/cm = 1000 mmhos/cm.
The above interrelation is approximate and is valid for a mixed salt solution and up to an electrical conductivity of 5 dS/m (Bhattacharya and Michael, 2003). For the solution of individual salts, the salt concentration in mg/L divided by the equivalent weight gives the salinity in meq/L. Thus, if 1000 mg of NaCl is dissolved in 1 L of pure water, the NaCl concentration will be 1000 mg/L or 1000 ppm or 1000/23 = 43.48 meq/L. The EC of this solution will be close to 5 dS/m or 5 mmhos/cm or 5000 mmhos/cm. The relation between the concentration of individual salts and the corresponding electrical conductivity is given for a number of commonly occurring and major salt components of soil in USDA (1954). Most plants grow best under a non-saline soil environment. However, every plant has a certain capacity to withstand saline soil condition to some extent. For most food crops, the upper limit of this tolerance is considered as an EC of 4 dS/m (Bhattacharya and Michael, 2003). If the EC of the soil solution exceeds this value, the plant may suffer irreversibly, thereby reducing crop yield. Electrical conductivity is an important parameter for describing the salinity status of water when such water is used for irrigation. It is equally important in drainage, because leaching of salt solutions by drainage reduces EC of the soil solution and makes the soil environment more conducive for plant growth. Electrical conductivity measurement and interpretation of the measured EC data are described in FAO Irrigation and Drainage Paper 57 (Rhoades et al., 1999). A useful and concise description is also given by van Hoorn and van Alphen (1994).
(2) pH
The soil reaction such as acidic, alkaline or neutral is expressed through pH. This notation stands for ‘power of Hydrogen’. Numerically, pH is defined as the logarithm of the reciprocal of the hydrogen ion concentration in the solution under consideration (i.e., soil solution). Acids are those which are the sources of H+ or H3O+ ions in aqueous solution, which can donate proton and which can accept pair of electrons. The bases (alkali) are those which are the sources of OH- in aqueous solution, which can accept proton and which can donate pair of electrons (Mishra and Mishra, 1996). In pure water, the concentrations of H3O+ or H+ and OH- ions are equal and is 1 ´ 10-7 moles per liter at 295 K (i.e., at 22 °C). Accordingly, the pH of pure water is log (1/10-7) = log 107 = 7.
A pH less than 7 represents an acidic soil solution and a pH greater than 7 represents an alkaline soil solution. The upper limit of pH is 14 [log (1/10-14) = 14] and the lower limit of pH is zero [log (1/100) = 0]. Though a pH of 7 is generally considered ideal for plant growth, most plant grow and yield quite well under a soil-water environment ranging from slightly acidic to slightly alkaline. Some plants (e.g., tea) prefer a slightly acidic soil condition than neutral, which is due to specific plant characteristics. If the chemical condition of a soil is beyond the tolerance capacity of a plant, remedial measures are required for improved plant growth and crop yield. The most important and widely adopted remedial measure is to add chemical amendment to the soil (e.g., lime to neutralize acidity and gypsum to neutralize the alkalinity) in presence of water and remove the resulting chemical products of the reaction from the soil-water-plant root system by leaching through drainage.
(3) Sodium Adsorption Ratio and Exchangeable Sodium Percent
For diagnosing, if a soil is seriously alkaline, one may adopt the soil quality parameter pH. Its measurement is done using a simple instrument or by titration method in the laboratory, or by a portable instrument in the field. Since alkalinity of a soil could be due to the presence of several bases in soil solution, all of which are not as harmful to the plants as Sodium, determination of sodium hazard becomes necessary. If the concentration of Sodium in the soil solution is more than the concentration of other major bases, then such a soil is usually not good for normal plant growth, and hence the excess Sodium has to be removed. A soil which is alkaline due to high presence of Sodium is also termed ‘Sodic Soil’. The proportion of sodium in relation to two other important bases, i.e., Calcium and Magnesium is expressed by the parameter known as Sodium Adsorption Ratio (SAR). SAR is defined as:
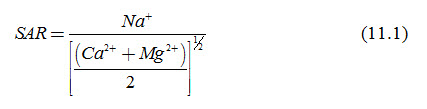
Where, SAR = Sodium Adsorption Ratio (meq/L)1/2, and Na+, Ca2+ and Mg2+ are concentrations of Sodium, Calcium and Magnesium cations in the saturation extract of the soil in meq/L.
Exchangeable Sodium Percent (ESP) is calculated by an approximate empirical relation, which is given as follows (USDA, 1954):

The above empirical relation between ESP and SAR is valid for a lower limit of SAR of 0.8542 (meq/L)1/2. At this value of SAR, the ESP becomes zero. At a value of SAR lower than this, the ESP becomes negative. Another important feature of the above relation is that for SAR values between 5 and 30, the estimated values of ESP do not differ much from the SAR values as shown in Table 11.2.
Table 11.2. Numerical correspondence between SAR and ESP values (Bhattacharya and Michael, 2003)
|
SAR (meq/L)1/2 |
1 |
5 |
10 |
15 |
20 |
25 |
30 |
35 |
40 |
45 |
50 |
|
ESP (%) |
0.2 |
5.8 |
12.0 |
17.3 |
22.3 |
26.5 |
30.4 |
33.4 |
36.9 |
39.8 |
42.4 |
The relation [Eqn. (11.2)] is not universal, as is the case for any empirical relation. This is due to the fact that the exchangeable Sodium is not necessarily the function of the relative proportions of Calcium, Magnesium and Sodium only (i.e., SAR) in the soil. Therefore, in a strict sense, the exchangeable Sodium percentage (ESP) is a ratio of exchangeable Sodium to the exchangeable total cations (i.e., Cation Exchange Capacity, CEC). To express in percentage, the ratio is multiplied by 100 to obtain ESP.
Note that for alkali soil characterization in drainage studies, the ESP, as defined in terms of SAR is normally sufficient (Bhattacharya and Michael, 2003). Moreover, in alkali soils, the interplay between Sodium, Calcium and Magnesium are generally more dominant. Therefore, to reduce Sodium hazard, the exchangeability of Sodium by the other two cations (mainly by Calcium) is a more important consideration. Nevertheless, the use of CEC is made while estimating the gypsum requirement for the reclamation of alkali soils.
In the semi-arid and arid regions, the rainfall is scarce and uncertain. The evapotranspirative demand of the atmosphere is, however, constantly very high due to high temperature and wind velocity. This mismatch between the demand and the supply of the water does not permit a favourable salt and water balance to develop in the soil. The soil solution is drawn towards the surface and the salts in it are precipitated in the surface soil layers when the water evaporates, turning the surface soil highly saline. In arid regions, Sodium, Magnesium, and Calcium salts are concentrated mainly in Chloride and Sulphate forms. In less arid regions, salt concentration is less and Sodium is found predominantly in Carbonate and Bi-carbonate forms. Such forms of Sodium favour the formation of sodic soils (Bhattacharya and Michael, 2003). Soil sodifies when the Sodium is adsorbed in the exchange complex. Since clay soil has much larger surface area per unit mass as compared to sandy soil, the former is more prone to sodification. The process of soil sodification includes desalinization in absence of enough divalent cations and with insufficient drainage, evaporation from the capillary rise of groundwater rich in NaHCO3 and Na2CO3, decomposition of Sodium Alumino Silicates, denitrification and sulphate reduction under anaerobic condition (e.g., in rice fields or waterlogged lands), irrigation with water of low salinity but with dominant HCO3 anions, and migration and accumulation of sodic salts in arid climate (Bhattacharya and Michael, 2003). Desalinization (reclamation of saline soil by leaching) in absence of enough divalent cations and with insufficient drainage also turns a land sodic (FAO, 2000).
11.3 Drainage of Irrigated Regions
11.3.1 Irrigation Water Quality vis-à-vis Soil salinity
In irrigated areas, soil salinization is mostly a post-irrigation development caused by seepage through unlined water conveyance network and adoption of faulty irrigation practices. Addition of salts through the application of poor quality irrigation water and a lack of proper drainage to dispose runoff during monsoon and control water table are the other factors causing soil salinity. Such risks are more in the semi-arid and arid regions of the world.
When water is used for irrigation, its chemical composition has a direct influence on the resulting chemical composition of the soil. Rainwater is the purest source of water and when used for irrigation, it does not produce chemical hazard (except the washing away and leaching of cations, which is a slow process). Other waters, i.e., canal water and groundwater contain salts in varying degree. Such water may be saline, with or without Sodium hazard. Accordingly, use of such waters in irrigation, turns the agricultural land saline or alkaline. As a thumb rule, most irrigated crops will tolerate dissolved salts in the irrigation water below 600 ppm (Bhattacharya and Michael, 2003). With the provision of drainage and leaching, most crops can tolerate irrigation water salinity up to 1500 ppm of dissolved salts (FAO, 2000). The groundwater quality also varies to a very great extent in different parts of India. Saline or high Sodium content groundwater is a major problem facing the irrigators in several states such as Punjab, Haryana, Rajasthan and Gujarat, to name a few important ones (Bhattacharya and Michael, 2003).
Acidic water in the natural water sources such as in rivers and streams is a local phenomenon. It happens over some specific reaches of these sources that pass through coal mine regions, as in the state of Meghalaya and parts of Bihar (Bhattacharya and Michael, 2003). The major cause of such occurrences is unplanned and unscientific human activities. This problem is common in the non-monsoon months when the river flow is low. However, if these waters are used for irrigation, it makes the soil acidic. Comprehensive descriptions of the methods of determination of important cations and anions in soil solutions can be found in USDA (1954), van Hoorn and van Alphen (1994), Rhoades et al. (1999) and Singh et al. (1999).
11.3.2 Salinity of Irrigation Water Sources: Indian Scenario
All irrigation water contains some dissolved salts from as low as 100 ppm to as high as 7500 ppm (Paliwal, 1972; Rhoades, 1974). The canal water qualities are generally the same as the waters of their parent rivers, unless it is contaminated while passing through a salt-affected area. River water with a salt concentration of 100 ppm contains 0.1 kg of salt in 1 m3 of water. A 30 cm water application to 1 ha of wheat field with such water will add 300 kg of salt in one hectare in one irrigation season. Table 11.3 presents the quantities of salt added to the soil by irrigating with waters of different salinity levels. Due to low surface irrigation efficiency, one has to apply water more than the irrigation requirement. Some excess water application is also needed to leach down the salts below the root zone, which is known as ‘leaching requirement’. This causes addition of more salt than the amount mentioned in Table 11.3.
Table 11.3. Salt addition to 1 ha of land due to application of 1 ha-cm irrigation water of different salinity levels
(Bhattacharya and Michael, 2003)
|
Sl. No. |
Salinity of Irrigation Water |
Salt Added (kg) |
|
|
TDS (mg/L) |
EC (dS/m) |
||
|
1 |
100 (Freshwater) |
0.156 |
10 |
|
2 |
500 (Freshwater) |
0.781 |
50 |
|
3 |
1000 (Marginal water) |
1.563 |
100 |
|
4 |
1500 (Marginal water) |
2.344 |
150 |
|
5 |
2000 (Brackish water) |
3.125 |
200 |
|
6 |
3000 (Brackish water) |
4.688 |
300 |
|
7 |
5000 (Brackish water) |
7.813 |
500 |
|
8 |
7000 (Saline water) |
10.938 |
700 |
|
9 |
10,000 (Saline water) |
15.625 |
1000 |
The water quality of the Indian rivers is generally good, except in the dry season in some of the rivers and when the river water gets contaminated by polluting water from different sources. Salient information on the quality of water of some of the Indian rivers is provided in Table 11.4. Note that the salt load due to irrigation will be over and above the salts already present in the soil. The salinity of the waters in canals fed from the rivers such as Ganga, Yamuna and Kosi ranges from 0.2 to 0.44 dS/m (128-282 ppm). In West Bengal, the canal water in 24 Parganas has salinity of 2.2 dS/m (1408 ppm) and in Howrah, the canal water salinity becomes as high as 12.6 dS/m (8064 ppm) when it gets mixed with tidal water (Bhattacharya and Michael, 2003).
Table 11.4. Salinity of water of some of the Indian rivers (Paliwal, 1972)
|
Sl. No. |
River |
Salinity of Water |
River |
Salinity of Water |
||
|
EC (dS/m) |
TDS (mg/L) |
EC (dS/m) |
TDS (mg/L) |
|||
|
1 |
Beas |
0.26 |
166 |
Kankawati |
1.70 |
1088 |
|
2 |
Brahmaputra |
0.18 |
115 |
Kosi |
0.14 |
90 |
|
3 |
Cauvery |
0.27 |
173 |
Krishna |
1.39 |
890 |
|
4 |
Chambal |
0.45 |
288 |
Mathal |
2.19 |
1402 |
|
5 |
Chandrabhaga |
3.54 |
2266 |
Narmada |
0.34 |
218 |
|
6 |
Gandak |
0.25 |
160 |
Rupen |
6.78 |
4339 |
|
7 |
Ganga |
0.27 |
173 |
Satlej |
0.33 |
211 |
|
8 |
Hagari |
1.64 |
1050 |
Sengar |
1.05 |
672 |
|
9 |
Hansthal |
0.60 |
384 |
Tapti |
0.55 |
352 |
|
10 |
Jangadia |
7.42 |
4749 |
Umai |
4.27 |
2733 |
|
11 |
Kali Nadi |
0.66 |
422 |
Yamuna |
0.65 |
416 |
Moreover, groundwater and runoff water stored in tanks which are used for irrigation have a great variation in their salinity and in many cases they are more saline than the river water or canal water. Salinity of runoff water collected in tanks in Uttar Pradesh varies from 1 to 6 dS/m. The well waters in most of the districts of Rajasthan have salinity greater than 3 dS/m and going up to 14 dS/m. The well waters in northern Gujarat have salinity ranging from 1 to 15 dS/m. These are in contrast to the groundwater salinity of less than 1 to 2 dS/m over most of Uttar Pradesh, except in Agra and Mathura regions where it is as high as 21 dS/m (Paliwal, 1972).
11.4 Drainage of Humid Regions
Humid regions receive annual rainfall in excess of 1000 mm, much of which occurs as heavy rains in short spells. About 80% of the annual rainfalls are received in less than 3 months and 80% of this occurs in about 8 to 10 rainfall events (Bhattacharya and Michael, 2003). In a region receiving 1500 mm annual rainfall, one may expect a rainfall of about 100 mm in one day and a major portion of this may occur in a few hours. The infiltration capacity of soils varies from 4 mm/h in clay loam soils to 40 mm/h in sandy soils (Bhattacharya and Michael, 2003). Under monsoon climatic conditions, the intensity of rainfall is usually much higher than the infiltration capacity of soils. Therefore, runoff is quite common in monsoon dominated regions. Drainage of humid regions is expected to dispose runoff from agricultural lands.
11.5 Drainage of Coastal Regions
While discussing the drainage problems in arid, semi-arid and humid regions, it is also important to discuss the drainage problems of coastal regions which call for a special attention. In India, the arable coastal land lies adjoining over 7000 km long coast line covering eight states, from West Bengal in the east to Gujarat in the west (Bhattacharya and Michael, 2003). Coastal tracts are often considered similar in terms of the constraints to agricultural production that is low. Perhaps, cultivation of rice and its low productivity are the only common features in the coastal regions. The determinants of agricultural production such as soil, rainfall, climate, rice varieties, cultural practices, and the nature and severity of drainage problems vary from place to place in coastal regions. Annual rainfall varies from 800 mm to more than 2700 mm. Soil texture ranges from medium to heavy. The soil-water pH varies from 3.5 in acid sulphate soils to 9.5 in saline sodic soils. The EC of soil extracts varies from 3 to 30 dS/m. The hydraulic conductivity varies from practically zero to 1.5 m/day and the infiltration rate varies from 0.1 to 20 mm/h (Bhattacharya and Michael, 2003).
Land drainage problems in the coastal regions arise due to restricted natural outflow in a flat land, silted and weed-infested drains, and higher downstream water level during high tides. A unique case is the low-lying paddy lands in acid-sulphate soils of coastal Kerala where the land elevation is 1-2 m below the mean sea level (Bhattacharya and Michael, 2003). The protective bunds built around the cultivated fields frequently breach, causing inflow of huge quantities of outside water to the paddy fields that must be drained. Also, lands are purposely kept under water fallow during the non-cultivated season to reduce acidity hazard. This water needs to be drained before the start of cultivation.
In general, the elevation of most of the coastal agricultural lands is only marginally higher than the sea level. When they suddenly receive a large quantity of water, which may be due to cyclonic storms (an annual event over much of the east coast), water release from upstream reservoirs or accumulation of runoff from higher reaches -- all of which are uncontrollable to a great extent, the water stagnates over the land (Bhattacharya and Michael, 2003). Quite often, water inundates the paddy plants completely and the situation may prolong which destroys the crop. Monsoon (kharif) cultivation in these areas becomes risky and can succeed only in some years when the magnitude of the incoming water from different sources is small. Seawater backflow during high tides is a recurring phenomenon in the coastal areas. This brings in large amount of dissolved salts, contaminating the local water bodies and the low-lying lands. Therefore, waterlogging and salinity are the two major problems faced by the farmers of the coastal regions (Bhattacharya and Michael, 2003). The dilution effect of the large volume of incoming freshwater during the monsoon season considerably reduces the salinity hazard in this season. Salinity problem is dominant during dry seasons due to high evapotranspiration, contaminated water in the local water bodies and the lack of adequate fresh surface water or groundwater. Thus, rice cultivation suffers during both monsoon (kharif) and winter (rabi) seasons.
References
Bhattacharya, A.K. and Michael, A.M. (2003). Land Drainage: Principles, Methods and Applications. Konark Publishers Pvt. Ltd., New Delhi, India.
FAO (2000). Land and Plant Nutrient Service. Global Network on Integrated Soil Management for Sustainable Use of Salt Affected Soils, FAO, Rome, Italy, http//www.org/ag/agl//agll/spush/topicl.htm.
Mishra, R.R. and Mishra, N. (1996). Understanding Chemistry through Numerical Problems. Arya Book Depot, New Delhi, India.
Paliwal, K.V. (1972). Irrigation with Saline Water. IARI Monograph No. 2, Water Technology Centre, IARI, New Delhi, India.
Rhoades, J.D. (1974). Drainage for Salinity Control. In: Jan Van Schilfgaarde (Editor), Drainage for Agriculture, Monograph No. 17, American Society of Agronomy, Madison, Wisconsin.
Rhoades, J.D., Chanduvi, F. and Lesch, S. (1999). Soil Salinity Assessment: Methods and Interpretation of Electrical Conductivity Measurement. FAO Irrigation and Drainage Paper 57, FAO, Rome, Italy.
Singh, D., Chhonkar, P.K. and Pandey, R.N. (1999). Soil Plant Water Analysis: A Methods Manual. Indian Agricultural Research Institute, New Delhi.
USDA (1954). Diagnosis and Improvement of Saline and Alkali Soils. USDA Handbook No. 60, U.S. Salinity Laboratory, United States Department of Agriculture (USDA), Washington DC.
van Hoorn, J.W. and van Alphen, J.G. (1994). Salinity Control. In: H.P. Ritzema (Editor-in-Chief), Drainage Principles and Applications, International Institute for Land Reclamation and Improvement (ILRI), ILRI Publication 16, Wageningen, The Netherlands, pp. 533-600.
Suggested Readings
Bhattacharya, A.K. and Michael, A.M. (2003). Land Drainage: Principles, Methods and Applications. Konark Publishers Pvt. Ltd., New Delhi, India.
Murty, V.V.N. and Jha, M.K. (2011). Land and Water Management Engineering. Sixth Edition, Kalyani Publishers, Ludhiana, India.
Ritzema (Editor-in-Chief) (1994). Drainage Principles and Applications. International Institute for Land Reclamation and Improvement (ILRI), ILRI Publication 16, Wageningen, The Netherlands.
Schwab, G.O., Fangmeier, D.D., Elliot, W.J. and Frevert, R.K. (2005). Soil and Water Conservation Engineering. Fourth Edition, John Wiley and Sons (Asia) Pte. Ltd., Singapore.
Smedema, L.K. and Rycroft, D.W. (1983). Land Drainage. Batsford Academic and Education Ltd., London.